Brain Tumor Res Treat.
2019 Oct;7(2):74-84. 10.14791/btrt.2019.7.e43.
The Korean Society for Neuro-Oncology (KSNO) Guideline for WHO Grade II Cerebral Gliomas in Adults: Version 2019.01
- Affiliations
-
- 1Division of Neurooncology and Department of Neurosurgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 2Department of Neurosurgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 3Department of Radiation Oncology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 4Department of Neurosurgery, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea.
- 5Department of Neurosurgery, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 6Department of Neurosurgery, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 7Department of Neurosurgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 8Department of Neurosurgery, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- 9Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 10Department of Radiation Oncology, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 11Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 12Division of Medical Oncology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 13Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 14Department of Neurosurgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 15Clinic of Pediatric Oncology, National Cancer Center, Goyang, Korea.
- 16Department of Neurosurgery, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- 17Department of Neurosurgery, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea.
- 18Department of Neurosurgery, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 19Department of Neurosurgery, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea. dschung@catholic.ac.kr
- 20Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- 21Department of Radiation Oncology, Ewha Women's University Mokdong Hospital, Ewha Women's University School of Medicine, Seoul, Korea.
- 22Department of Neurology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 23Department of Radiation Oncology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 24Department of Pathology, Seoul St. Marry's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 25Department of Neurosurgery, Bundang CHA Medical Center, CHA University, Seongnam, Korea.
- 26Department of Neurosurgery, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Gwangju, Korea.
- 27Department of Pediatrics, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 28Department of Radiology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 29Department of Pediatrics, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 30Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dh8lim@skku.edu
- KMID: 2461179
- DOI: http://doi.org/10.14791/btrt.2019.7.e43
Abstract
- BACKGROUND
There was no practical guideline for the management of patients with central nervous system tumor in Korea for many years. Thus, the Korean Society for Neuro-Oncology (KSNO), a multidisciplinary academic society, has developed the guideline for glioblastoma. Subsequently, the KSNO guideline for World Health Organization (WHO) grade II cerebral glioma in adults is established.
METHODS
The Working Group was composed of 35 multidisciplinary medical experts in Korea. References were identified by searching PubMed, MEDLINE, EMBASE, and Cochrane CENTRAL databases using specific and sensitive keywords as well as combinations of keywords regarding diffuse astrocytoma and oligodendroglioma of brain in adults.
RESULTS
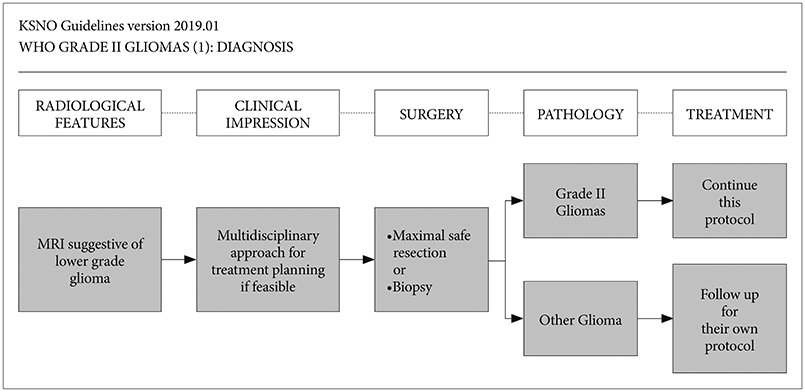
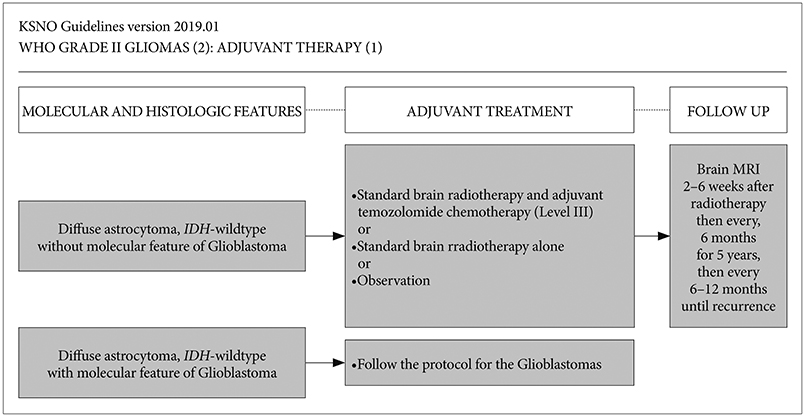
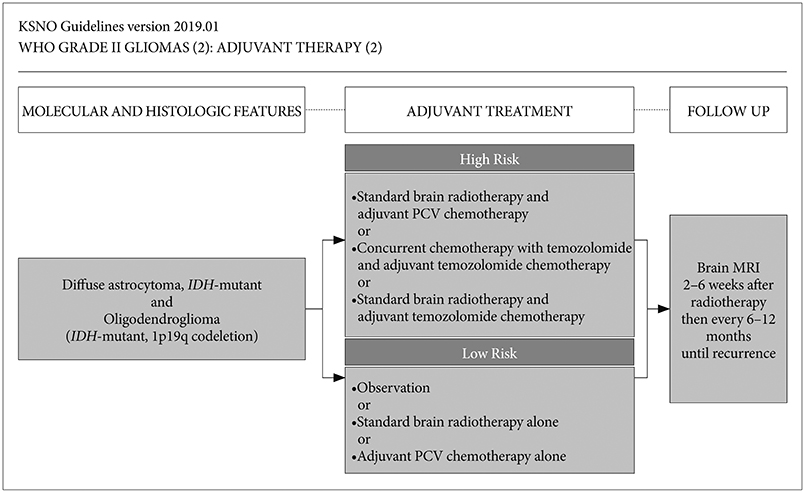
Whenever radiological feature suggests lower grade glioma, the maximal safe resection if feasible is recommended globally. After molecular and histological examinations, patients with diffuse astrocytoma, isocitrate dehydrogenase (IDH)-wildtype without molecular feature of glioblastoma should be primarily treated by standard brain radiotherapy and adjuvant temozolomide chemotherapy (Level III) while those with molecular feature of glioblastoma should be treated following the protocol for glioblastomas. In terms of patients with diffuse astrocytoma, IDH-mutant and oligodendroglioma (IDH-mutant and 1p19q codeletion), standard brain radiotherapy and adjuvant PCV (procarbazine+lomustine+vincristine) combination chemotherapy should be considered primarily for the high-risk group while observation with regular follow up should be considered for the low-risk group.
CONCLUSION
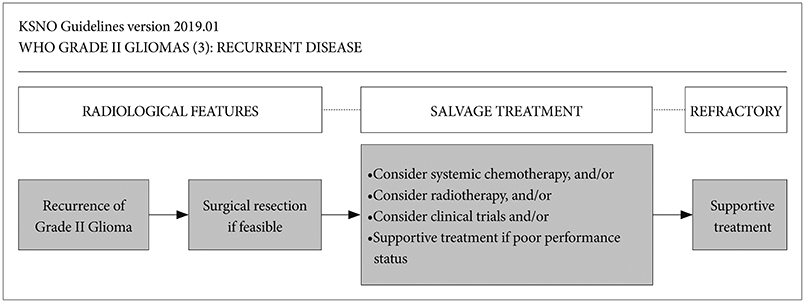
The KSNO's guideline recommends that WHO grade II gliomas should be treated by maximal safe resection, if feasible, followed by radiotherapy and/or chemotherapy according to molecular and histological features of tumors and clinical characteristics of patients.
MeSH Terms
Figure
Cited by 1 articles
-
Korean Brain Tumor Society Consensus Review for the Practical Recommendations on Glioma Management in Korea
Chul-Kee Park, Jong Hee Chang
J Korean Neurosurg Soc. 2023;66(3):308-315. doi: 10.3340/jkns.2023.0046.
Reference
-
1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018; 20:suppl_4. iv1–iv86.
Article2. Dho YS, Jung KW, Ha J, et al. An updated nationwide epidemiology of primary brain tumors in Republic of Korea, 2013. Brain Tumor Res Treat. 2017; 5:16–23.
Article3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–820.
Article4. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO classification of tumours of the central nervous system, revised. 4th ed. Lyon: International Agency for Research on Cancer;2016.5. Van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician's perspective. Acta Neuropathol. 2010; 120:297–304.
Article6. Malzkorn B, Reifenberger G. Practical implications of integrated glioma classification according to the World Health Organization classification of tumors of the central nervous system 2016. Curr Opin Oncol. 2016; 28:494–501.
Article7. Weller M, Wick W, Aldape K, et al. Glioma. Nat Rev Dis Primers. 2015; 1:15017.
Article8. Louis DN, Perry A, Burger P, et al. International Society of Neuropathology--Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014; 24:429–435.
Article9. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018; 136:805–810.
Article10. Koo T, Lim DH, Seol HJ, et al. Impact of adjuvant treatments on survival in Korean patients with WHO grade II gliomas: KNOG 15-02 and KROG 16-04 intergroup study. J Neurooncol. 2018; 140:445–455.
Article11. Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002; 20:2267–2276.
Article12. Van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005; 366:985–990.
Article13. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016; 374:1344–1355.
Article14. Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016; 17:1521–1532.
Article15. Kim YZ, Kim CY, Lim J, et al. The Korean Society for Neuro-Oncology (KSNO) Guideline for Glioblastomas: Version 2018.01. Brain Tumor Res Treat. 2019; 7:1–9.
Article16. Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017; 35:2439–2449.
Article17. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, central nervous system cancers. Version 1.2019. Accessed August 1, 2019. at https://www.nccn.org/professionals/physician_gls/default.aspx#cns.18. Van den Bent MJ. Practice changing mature results of RTOG study 9802: another positive PCV trial makes adjuvant chemotherapy part of standard of care in low-grade glioma. Neuro Oncol. 2014; 16:1570–1574.
Article19. Buckner JC, Gesme D Jr, O'Fallon JR, et al. Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J Clin Oncol. 2003; 21:251–255.
Article20. Soffietti R, Rudà R, Bradac GB, Schiffer D. PCV chemotherapy for recurrent oligodendrogliomas and oligoastrocytomas. Neurosurgery. 1998; 43:1066–1073.
Article21. Shaw EG, Wang M, Coons SW, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. 2012; 30:3065–3070.
Article22. Fisher BJ, Hu C, Macdonald DR, et al. Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: preliminary results of Radiation Therapy Oncology Group 0424. Int J Radiat Oncol Biol Phys. 2015; 91:497–504.
Article23. Gwak HS, Yee GT, Park CK, et al. Temozolomide salvage chemotherapy for recurrent anaplastic oligodendroglioma and oligo-astrocytoma. J Korean Neurosurg Soc. 2013; 54:489–495.
Article24. Gorlia T, Wu W, Wang M, et al. New validated prognostic models and prognostic calculators in patients with low-grade gliomas diagnosed by central pathology review: a pooled analysis of EORTC/RTOG/NCCTG phase III clinical trials. Neuro Oncol. 2013; 15:1568–1579.
Article25. Yeaney GA, Brat DJ. What every neuropathologist needs to know: update on cIMPACT-NOW. J Neuropathol Exp Neurol. 2019; 78:294–296.
Article26. Avila EK, Chamberlain M, Schiff D, et al. Seizure control as a new metric in assessing efficacy of tumor treatment in low-grade glioma trials. Neuro Oncol. 2017; 19:12–21.
Article27. Van den Bent MJ, Smits M, Kros JM, Chang SM. Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol. 2017; 35:2394–2401.
Article28. Louis DN, Aldape K, Brat DJ, et al. Announcing cIMPACT-NOW: the consortium to inform molecular and practical approaches to CNS Tumor taxonomy. Acta Neuropathol. 2017; 133:1–3.
Article29. Louis DN, Giannini C, Capper D, et al. cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol. 2018; 135:639–642.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Overview of Practical Guidelines for Gliomas by KSNO, NCCN, and EANO
- The Korean Society for Neuro-Oncology (KSNO) Guideline for WHO Grade III Cerebral Gliomas in Adults: Version 2019.01
- The Korean Society for Neuro-Oncology (KSNO) Guideline for the Management of Brain Tumor Patients During the Crisis Period: A Consensus Survey About Specific Clinical Scenarios (Version 2023.1)
- Perfusion MR Imaging in Gliomas: Comparison with Histologic Tumor Grade
- Yesterdays, Todays, and Tomorrows—Korean Society for Pediatric Neuro-Oncology*