Molecular Correlates and Nuclear Features of Encapsulated Follicular-Patterned Thyroid Neoplasms
- Affiliations
-
- 1Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Pathology, Kameda Medical Center, Kamogawa, Japan
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Pathology, Ajou University School of Medicine, Suwon, Korea
- 5Department of Pathology, Jiangsu Institute of Nuclear Medicine, Wuxi, China
- 6Department of Pathology, Sixth People’s Hospital, Shanghai Jiao Tong University, Shanghai, China
- 7Department of Pathology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 8Department of Pathology, Taipei Veterans General Hospital, Taipei, Taiwan
- 9Department of Diagnostic Pathology and Cytology, Kuma Hospital, Kobe, Japan
- 10Division of Diagnostic Pathology, Keio University Hospital, Tokyo, Japan
- 11Department of Pathology and Thyroid Disease Center, Izumi City General Hospital, Izumi, Japan
- KMID: 2513296
- DOI: http://doi.org/10.3803/EnM.2020.860
Abstract
- Background
Assessing nuclear features is diagnostically challenging in the aspect of thyroid pathology. The aim of this study was to determine whether pathologists could distinguish BRAF-like and RAS-like nuclear features morphologically and identify morphological features to differentiate thyroid tumors with RAS-like mutations from encapsulated papillary thyroid carcinoma (PTC) with predominant follicular growth and BRAFV600E mutation.
Methods
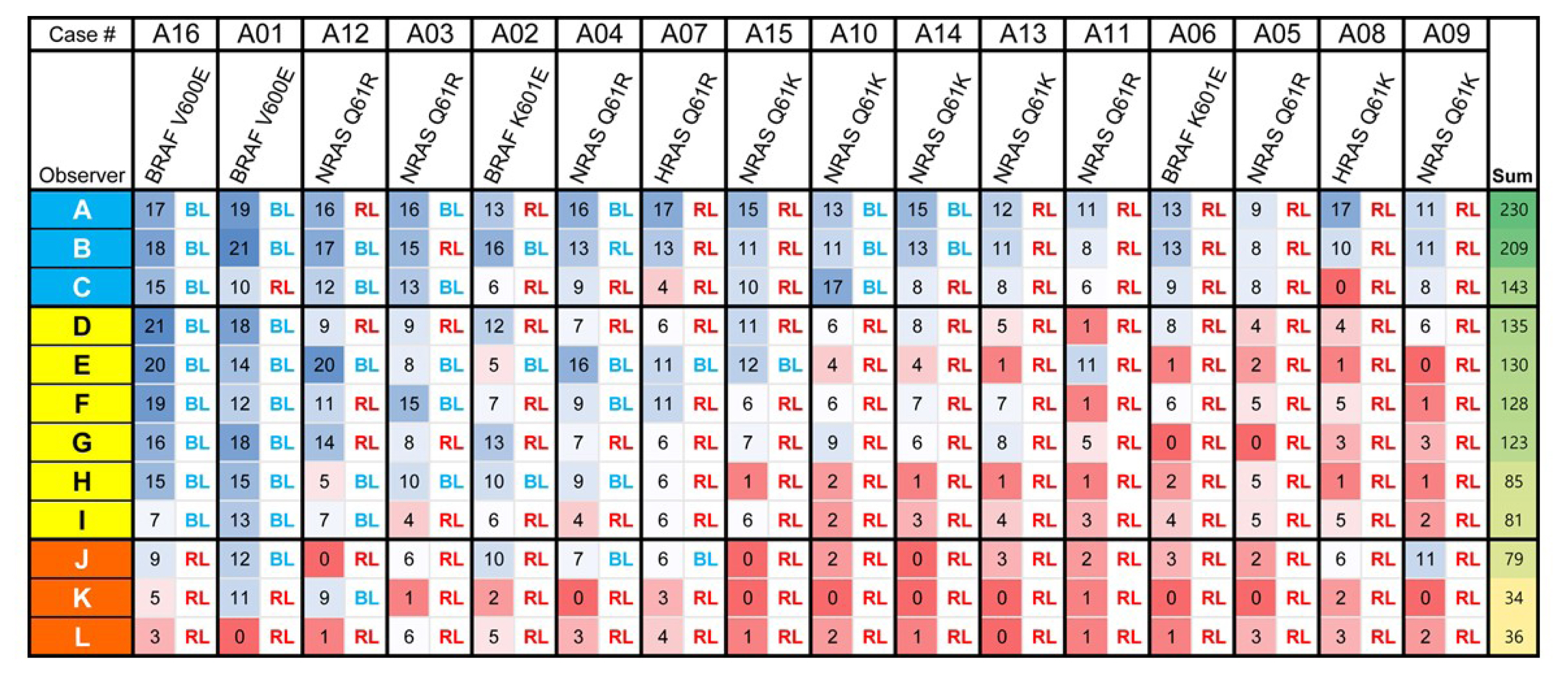
Representative whole slide images of 16 encapsulated thyroid tumors with predominant follicular growth were reviewed by 12 thyroid pathologists using a web browser-based image viewer. Total nuclear score was calculated from semi-quantitatively scored eight nuclear features. The molecular profile of RAS and BRAF genes was determined by Sanger sequencing.
Results
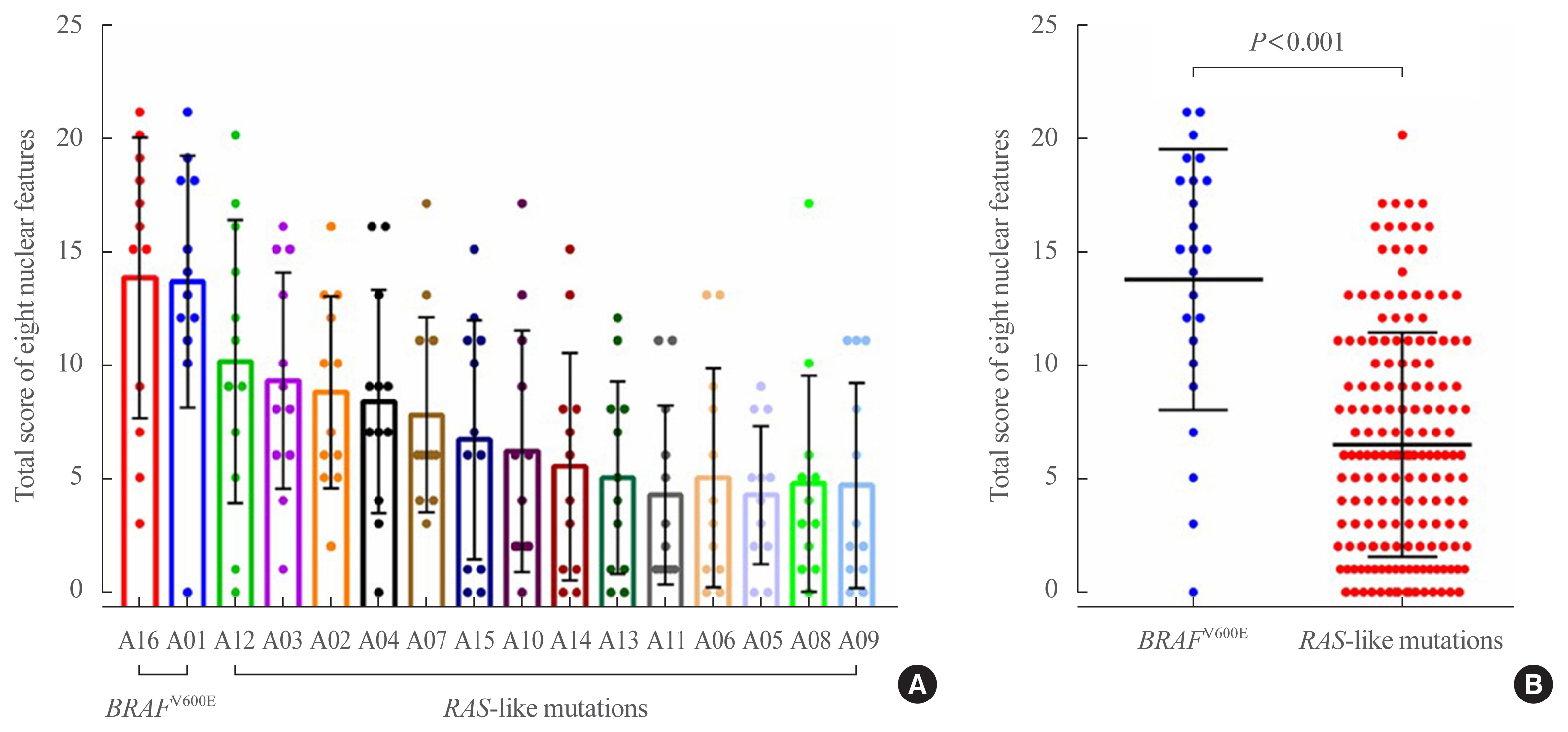
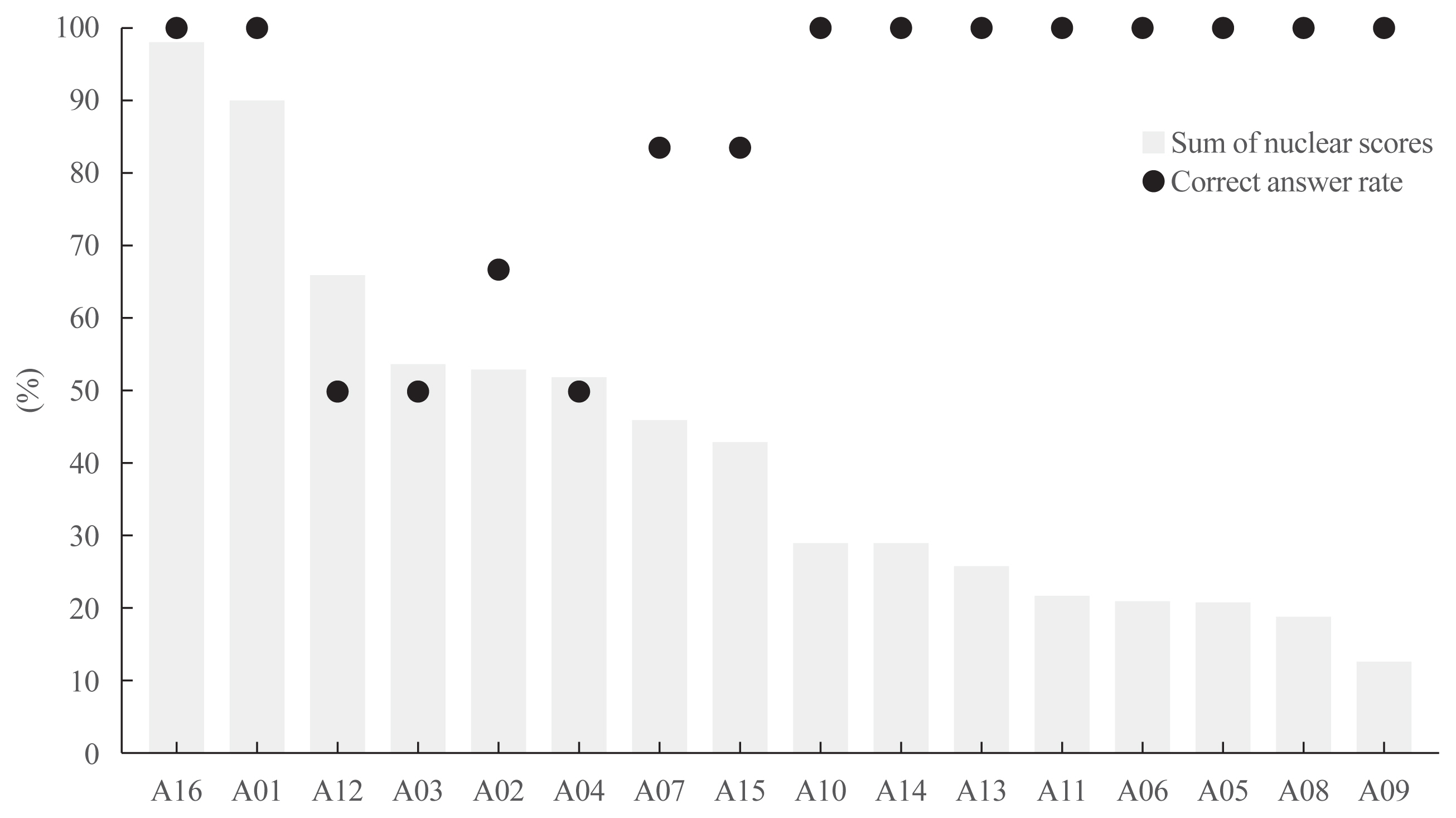
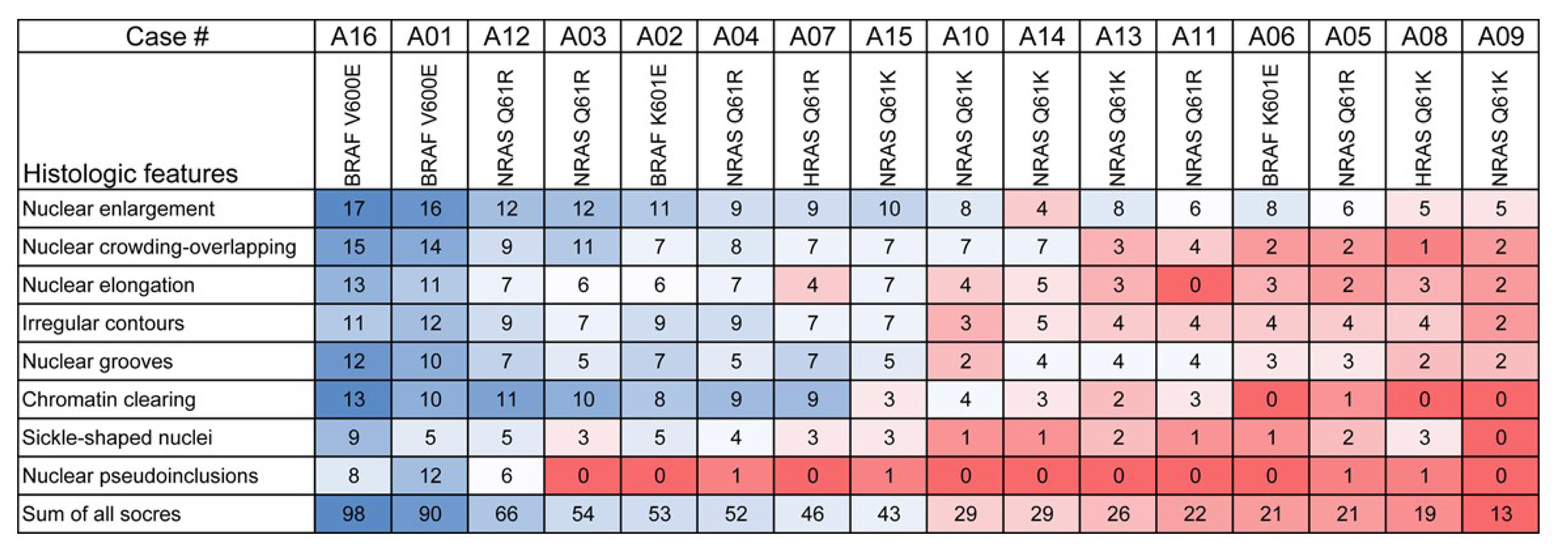
Total nuclear score ranging 0 to 24 could differentiate BRAF-like tumors from RAS-like tumors with a cut-off value of score 14. The interobserver agreement was the highest for the assessment of nuclear pseudoinclusions (NPIs) but the lowest for nuclear elongation and sickle-shaped nuclei. NPIs were found in tumors with BRAFV600E mutation, but not in tumors with RAS-like mutations. Total nuclear scores were significantly higher for tumors with BRAFV600E than for those with RAS-like mutations (P<0.001).
Conclusion
Our results suggest that NPIs and high nuclear scores have diagnostic utility as rule-in markers for differentiating PTC with BRAFV600E mutation from benign or borderline follicular tumors with RAS-like mutations. Relaxation of rigid criteria for nuclear features resulted in an overdiagnosis of PTC. Immunostaining or molecular testing for BRAFV600E mutation is a useful adjunct for cases with high nuclear scores to identify true PTC.
Keyword
Figure
Cited by 4 articles
-
Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach
Chan Kwon Jung, Andrey Bychkov, Kennichi Kakudo
Endocrinol Metab. 2022;37(5):703-718. doi: 10.3803/EnM.2022.1553.The Asian Thyroid Working Group, from 2017 to 2023
Kennichi Kakudo, Chan Kwon Jung, Zhiyan Liu, Mitsuyoshi Hirokawa, Andrey Bychkov, Huy Gia Vuong, Somboon Keelawat, Radhika Srinivasan, Jen-Fan Hang, Chiung-Ru Lai
J Pathol Transl Med. 2023;57(6):289-304. doi: 10.4132/jptm.2023.10.04.Small Multi-Gene DNA Panel Can Aid in Reducing the Surgical Resection Rate and Predicting the Malignancy Risk of Thyroid Nodules
Moon Young Oh, Hye-Mi Choi, Jinsun Jang, Heejun Son, Seung Shin Park, Minchul Song, Yoo Hyung Kim, Sun Wook Cho, Young Jun Chai, Woosung Chung, Young Joo Park
Endocrinol Metab. 2024;39(5):777-792. doi: 10.3803/EnM.2024.2034.Diagnostic challenges in the assessment of thyroid neoplasms using nuclear features and vascular and capsular invasion: a multi-center interobserver agreement study
Agnes Stephanie Harahap, Mutiah Mutmainnah, Maria Francisca Ham, Dina Khoirunnisa, Abdillah Hasbi Assadyk, Husni Cangara, Aswiyanti Asri, Diah Prabawati Retnani, Fairuz Quzwain, Hasrayati Agustina, Hermawan Istiadi, Indri Windarti, Krisna Murti, Muhammad Takbir, Ni Made Mahastuti, Nila Kurniasari, Nungki Anggorowati, Pamela Abineno, Yulita Pundewi Setyorini, Kennichi Kakudo
J Pathol Transl Med. 2024;58(6):299-309. doi: 10.4132/jptm.2024.07.25.
Reference
-
1. Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumors of endocrine organs. 4th ed. Lyon: International Agency for Research on Cancer (IARC);2017. p. 65–91.2. Zhu Y, Li Y, Jung CK, Song DE, Hang JF, Liu Z, et al. Histopathologic assessment of capsular invasion in follicular thyroid neoplasms-an observer variation study. Endocr Pathol. 2020; 31:132–40.
Article3. Liu Z, Bychkov A, Jung CK, Hirokawa M, Sui S, Hong S, et al. Interobserver and intraobserver variation in the morphological evaluation of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Pathol Int. 2019; 69:202–10.
Article4. Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016; 2:1023–9.
Article5. Nikiforov YE, Baloch ZW, Hodak SP, Giordano TJ, Lloyd RV, Seethala RR, et al. Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillarylike nuclear features. JAMA Oncol. 2018; 4:1125–6.
Article6. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159:676–90.7. Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016; 375:1054–67.
Article8. Tirro E, Martorana F, Romano C, Vitale SR, Motta G, Di Gregorio S, et al. Molecular alterations in thyroid cancer: from bench to clinical practice. Genes (Basel). 2019; 10:709.
Article9. Paulson VA, Shivdasani P, Angell TE, Cibas ES, Krane JF, Lindeman NI, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features accounts for more than half of “carcinomas” harboring RAS mutations. Thyroid. 2017; 27:506–11.
Article10. Kakudo K. Thyroid FNA cytology. 2nd ed. Singapore: Springer Nature Singapore Pte Ltd;2019. Chapter 21, Nuclear features of papillary thyroid carcinoma (BRAF-like tumors), noninvasive follicular thyroid neoplasm with papillary-like nuclear features (RAS-like tumors), and follicular adenoma/follicular thyroid carcinoma (RAS-like tumors). p. 173–9.11. Hirokawa M, Carney JA, Goellner JR, DeLellis RA, Heffess CS, Katoh R, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002; 26:1508–14.
Article12. Rosai J. The encapsulated follicular variant of papillary thyroid carcinoma: back to the drawing board. Endocr Pathol. 2010; 21:7–11.
Article13. Bychkov A, Jung CK, Liu Z, Kakudo K. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice: perspectives for surgical pathology and cytopathology. Endocr Pathol. 2018; 29:276–88.
Article14. Bychkov A, Keelawat S, Agarwal S, Jain D, Jung CK, Hong S, et al. Impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the Bethesda system for reporting thyroid cytopathology: a multi-institutional study in five Asian countries. Pathology. 2018; 50:411–7.
Article15. Hirokawa M, Higuchi M, Suzuki A, Hayashi T, Kuma S, Miyauchi A. Prevalence and diagnostic significance of noninvasive follicular thyroid neoplasm with papillary-like nuclear features among tumors previously diagnosed as follicular adenoma: a single-institutional study in Japan. Endocr J. 2020; 67:1071–5.
Article16. Zajkowska K, Kopczynski J, Gozdz S, Kowalska A. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a problematic entity. Endocr Connect. 2020; 9:R47–58.
Article17. Cho U, Mete O, Kim MH, Bae JS, Jung CK. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol. 2017; 30:810–25.
Article18. Jung CK, Kim Y, Jeon S, Jo K, Lee S, Bae JS. Clinical utility of EZH1 mutations in the diagnosis of follicular-patterned thyroid tumors. Hum Pathol. 2018; 81:9–17.
Article19. Bychkov A, Kakudo K, Hong S. Current practices of thyroid fine-needle aspiration in Asia: a missing voice. J Pathol Transl Med. 2017; 51:517–20.
Article20. Jung CK, Hong S, Bychkov A, Kakudo K. The use of fine-needle aspiration (FNA) cytology in patients with thyroid nodules in Asia: a brief overview of studies from the Working Group of Asian Thyroid FNA Cytology. J Pathol Transl Med. 2017; 51:571–8.
Article21. Rossi ED, Bizzarro T, Martini M, Capodimonti S, Fadda G, Larocca LM, et al. Morphological parameters able to predict BRAF(V600E) mutated malignancies on thyroid fine-needle aspiration cytology: our institutional experience. Cancer Cytopathol. 2014; 122:883–91.22. Legesse T, Parker L, Heath J, Staats PN. Distinguishing non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) from classic and invasive follicular-variant papillary thyroid carcinomas based on cytologic features. J Am Soc Cytopathol. 2019; 8:11–7.
Article23. Mahajan S, Agarwal S, Kocheri N, Jain D, Mathur SR, Iyer VK. Cytopathology of non-invasive follicular thyroid neoplasm with papillary-like nuclear features: a comparative study with similar patterned papillary thyroid carcinoma variants. Cytopathology. 2018; 29:233–40.
Article24. Arora SK, Dey P. Intranuclear peudoinclusions: morphology, pathogenesis, and significance. Diagn Cytopathol. 2012; 40:741–4.
Article25. Ip YT, Dias Filho MA, Chan JK. Nuclear inclusions and pseudoinclusions: friends or foes of the surgical pathologist? Int J Surg Pathol. 2010; 18:465–81.
Article26. Das DK. Intranuclear cytoplasmic inclusions in fine-needle aspiration smears of papillary thyroid carcinoma: a study of its morphological forms, association with nuclear grooves, and mode of formation. Diagn Cytopathol. 2005; 32:264–8.
Article27. Alves VA, Kakudo K, LiVolsi V, Lloyd RV, Nikiforov YE, Nose V, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): achieving better agreement by refining diagnostic criteria. Clinics (Sao Paulo). 2018; 73:e576.
Article28. Thompson LD, Poller DN, Kakudo K, Burchette R, Nikiforov YE, Seethala RR. An international interobserver variability reporting of the nuclear scoring criteria to diagnose noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a validation study. Endocr Pathol. 2018; 29:242–9.
Article29. Choi JE, Bae JS, Lim DJ, Jung SL, Jung CK. Atypical histiocytoid cells and multinucleated giant cells in fine-needle aspiration cytology of the thyroid predict lymph node metastasis of papillary thyroid carcinoma. Cancers (Basel). 2019; 11:816.
Article30. Selvaggi SM. The presence of multinucleated giant cells: noninvasive follicular thyroid neoplasm with papillary-like nuclear features vs the follicular variant of papillary thyroid carcinoma. Diagn Cytopathol. 2019; 47:1007–10.
Article31. Brooks E, Simmons-Arnold L, Naud S, Evans MF, Elhosseiny A. Multinucleated giant cells’ incidence, immune markers, and significance: a study of 172 cases of papillary thyroid carcinoma. Head Neck Pathol. 2009; 3:95–9.
Article32. Dvorak K, Aggeler B, Palting J, McKelvie P, Ruszkiewicz A, Waring P. Immunohistochemistry with the anti-BRAF V600E (VE1) antibody: impact of pre-analytical conditions and concordance with DNA sequencing in colorectal and papillary thyroid carcinoma. Pathology. 2014; 46:509–17.
Article33. Singarayer R, Mete O, Perrier L, Thabane L, Asa SL, Van Uum S, et al. A systematic review and meta-analysis of the diagnostic performance of BRAFV600E immunohistochemistry in thyroid histopathology. Endocr Pathol. 2019; 30:201–18.
Article34. Choden S, Keelawat S, Jung CK, Bychkov A. VE1 immunohistochemistry improves the limit of genotyping for detecting BRAFV600E mutation in papillary thyroid cancer. Cancers (Basel). 2020; 12:596.
Article35. Bychkov A, Hirokawa M, Jung CK, Liu Z, Zhu Y, Hong SW, et al. Low rate of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Thyroid. 2017; 27:983–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Understanding Neoplasm of Uncertain or Unknown Behavior of the Thyroid in Korean Clinical Practice
- Pathologic basis of the sonographic differences between thyroid cancer and noninvasive follicular thyroid neoplasm with papillary-like nuclear features
- Immunohistochemical and Molecular Characteristics of Follicular Patterned Thyroid Nodules with Incomplete Nuclear Features of Papillary Thyroid Carcinoma
- Clinicoradiological Characteristics in the Differential Diagnosis of Follicular-Patterned Lesions of the Thyroid: A Multicenter Cohort Study
- Reevaluating diagnostic categories and associated malignancy risks in thyroid core needle biopsy