J Pathol Transl Med.
2023 Jul;57(4):208-216. 10.4132/jptm.2023.06.20.
Reevaluating diagnostic categories and associated malignancy risks in thyroid core needle biopsy
- Affiliations
-
- 1Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2544328
- DOI: http://doi.org/10.4132/jptm.2023.06.20
Abstract
- As the application of core needle biopsy (CNB) in evaluating thyroid nodules rises in clinical practice, the 2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules have officially recognized its value for the first time. CNB procures tissue samples preserving both histologic structure and cytologic detail, thereby supplying substantial material for an accurate diagnosis and reducing the necessity for repeated biopsies or subsequent surgical interventions. The current review introduces the risk of malignancy within distinct diagnostic categories, emphasizing the implications of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on these malignancy risks. Prior research has indicated diagnostic challenges associated with follicular-patterned lesions, resulting in notable variation within indeterminate diagnostic categories. The utilization of mutation-specific immunostaining in CNB enhances the accuracy of lesion classification. This review underlines the essential role of a multidisciplinary approach in diagnosing follicular-patterned lesions and the potential of mutation-specific immunostaining to strengthen diagnostic consensus and inform patient management decisions.
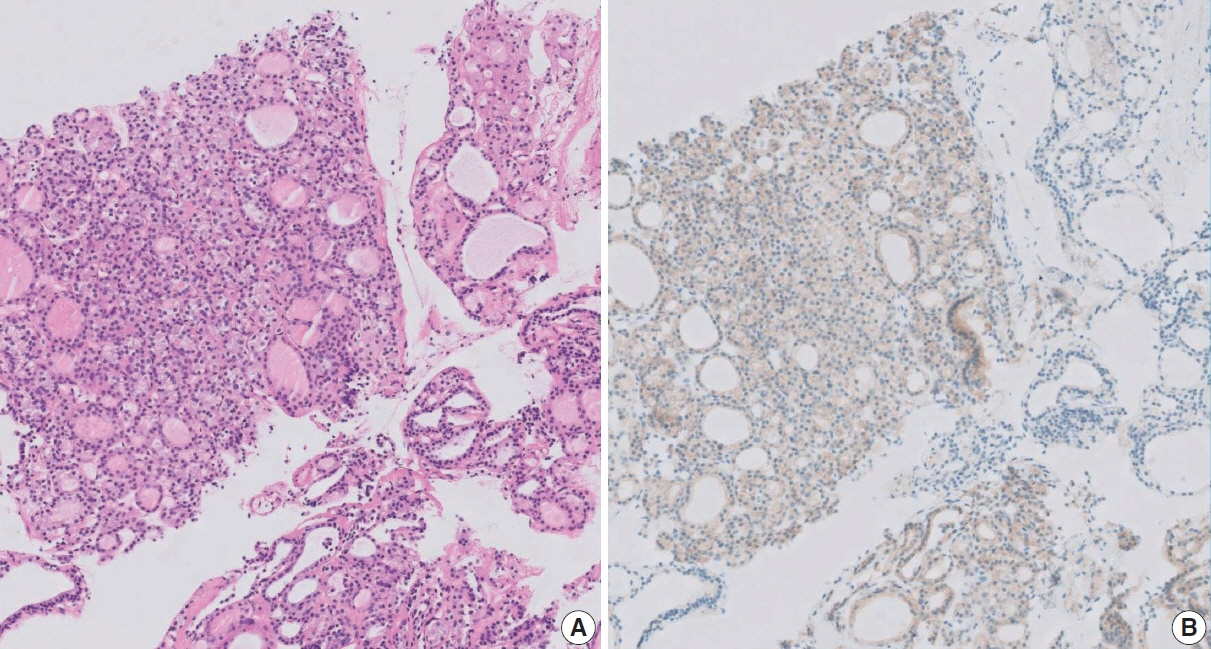
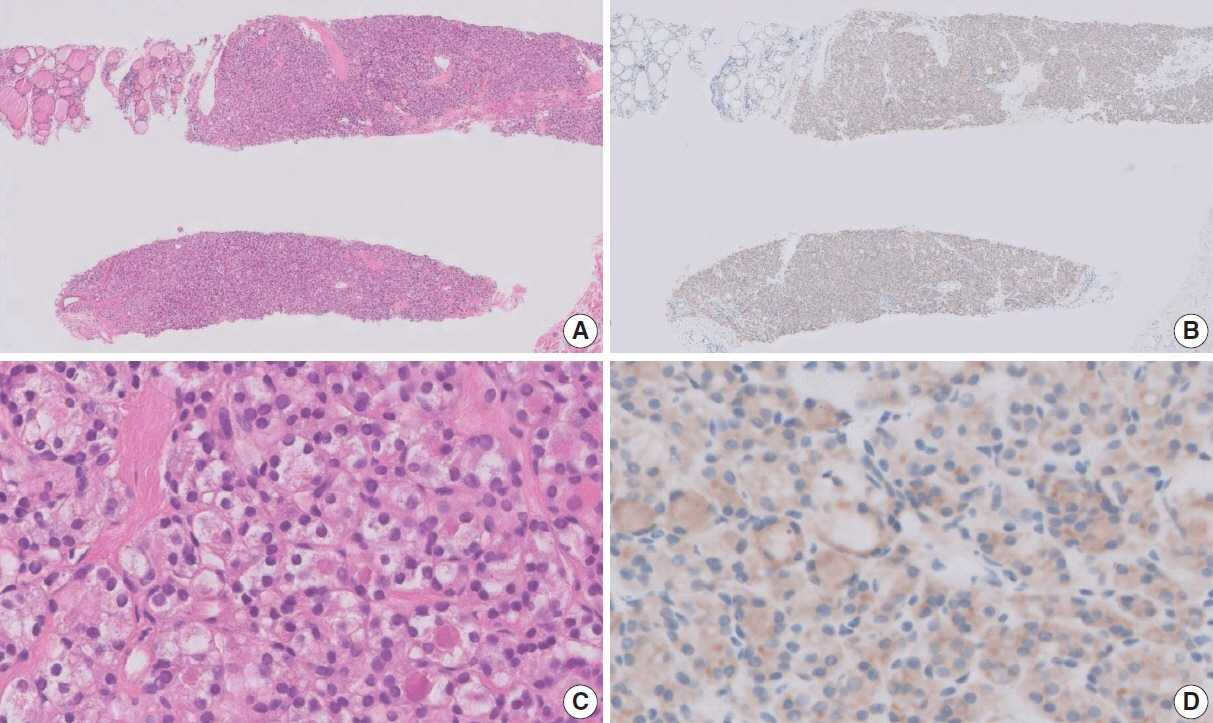
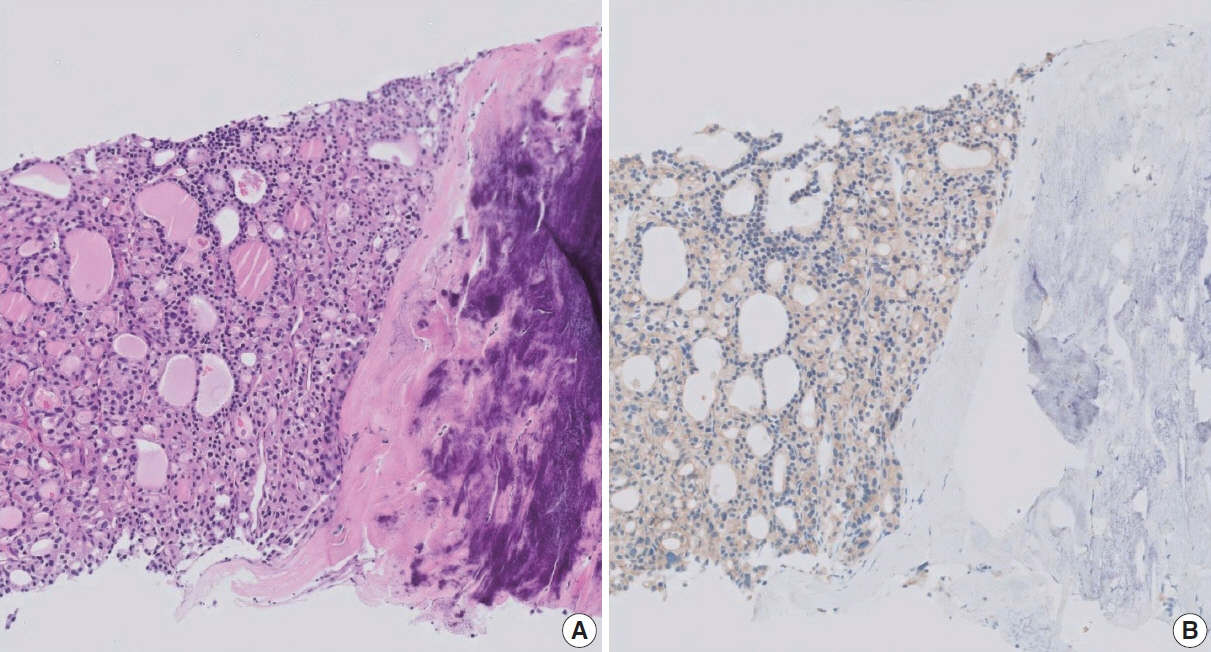
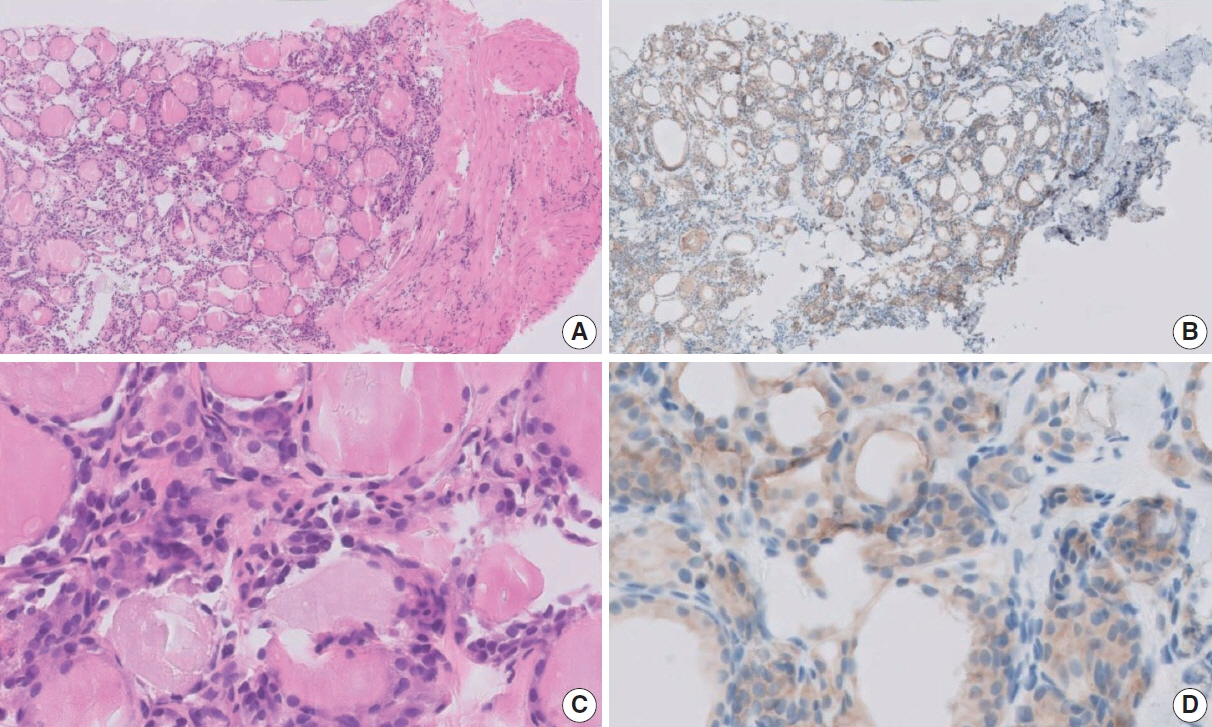
Figure
Cited by 1 articles
-
The Diagnostic Role of Repeated Biopsy of Thyroid Nodules with Atypia of Undetermined Significance with Architectural Atypia on Core-Needle Biopsy
Hye Hyeon Moon, Sae Rom Chung, Young Jun Choi, Tae-Yon Sung, Dong Eun Song, Tae Yong Kim, Jeong Hyun Lee, Jung Hwan Baek
Endocrinol Metab. 2024;39(2):300-309. doi: 10.3803/EnM.2023.1818.
Reference
-
References
1. Na DG, Baek JH, Jung SL, et al. Core needle biopsy of the thyroid: 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology. Korean J Radiol. 2017; 18:217–37.2. Liu N, Meng Z, Jia Q, et al. Ultrasound-guided core needle biopsy for differential diagnosis of thyroid nodules: a systematic review and meta-analysis. Mol Clin Oncol. 2017; 6:825–32.3. Trimboli P, Nasrollah N, Guidobaldi L, et al. The use of core needle biopsy as first-line in diagnosis of thyroid nodules reduces false negative and inconclusive data reported by fine-needle aspiration. World J Surg Oncol. 2014; 12:61.4. Suh CH, Baek JH, Lee JH, et al. The role of core-needle biopsy as a first-line diagnostic tool for initially detected thyroid nodules. Thyroid. 2016; 26:395–403.5. Kim HC, Kim YJ, Han HY, et al. First-line use of core needle biopsy for high-yield preliminary diagnosis of thyroid nodules. AJNR Am J Neuroradiol. 2017; 38:357–63.6. Zhang M, Zhang Y, Fu S, Lv F, Tang J. Thyroid nodules with suspicious ultrasound findings: the role of ultrasound-guided core needle biopsy. Clin Imaging. 2014; 38:434–8.7. Ahn HS, Youn I, Na DG, Kim SJ, Lee MY. Diagnostic performance of core needle biopsy as a first-line diagnostic tool for thyroid nodules according to ultrasound patterns: Comparison with fine needle aspiration using propensity score matching analysis. Clin Endocrinol (Oxf). 2021; 94:494–503.8. Kim M, Jeon S, Jung CK. Preoperative risk stratification of follicular-patterned thyroid lesions on core needle biopsy by histologic subtyping and RAS variant-specific immunohistochemistry. Endocr Pathol. 2023; 34:247–56.9. Jung CK, Baek JH, Na DG, Oh YL, Yi KH, Kang HC. 2019 Practice guidelines for thyroid core needle biopsy: a report of the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association. J Pathol Transl Med. 2020; 54:64–86.10. Jung CK, Min HS, Park HJ, et al. Pathology reporting of thyroid core needle biopsy: a proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group. J Pathol Transl Med. 2015; 49:288–99.11. Park YJ, Lee EK, Song YS, et al. 2023 Korean Thyroid Association management guidelines for patients with thyroid nodules. Int J Thyroidol. 2023; 16:1–31.12. Choe J, Baek JH, Park HS, Choi YJ, Lee JH. Core needle biopsy of thyroid nodules: outcomes and safety from a large single-center single-operator study. Acta Radiol. 2018; 59:924–31.13. Kim K, Bae JS, Kim JS, Jung SL, Jung CK. Diagnostic performance of thyroid core needle biopsy using the revised reporting system: comparison with fine needle aspiration cytology. Endocrinol Metab (Seoul). 2022; 37:159–69.14. Na HY, Woo JW, Moon JH, et al. Preoperative diagnostic categories of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in thyroid core needle biopsy and its impact on risk of malignancy. Endocr Pathol. 2019; 30:329–39.15. Ahn SH, Park SY, Choi SI. Comparison of consecutive results from fine needle aspiration and core needle biopsy in thyroid nodules. Endocr Pathol. 2017; 28:332–8.16. Yoon RG, Baek JH, Lee JH, et al. Diagnosis of thyroid follicular neoplasm: fine-needle aspiration versus core-needle biopsy. Thyroid. 2014; 24:1612–7.17. Chung SR, Baek JH, Lee JH, et al. Risk of malignancy according to the sub-classification of atypia of undetermined significance and suspicious follicular neoplasm categories in thyroid core needle biopsies. Endocr Pathol. 2019; 30:146–54.18. Na HY, Park SY. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: its updated diagnostic criteria, preoperative cytologic diagnoses and impact on the risk of malignancy. J Pathol Transl Med. 2022; 56:319–25.19. Ahn SH. Usage and diagnostic yield of fine-needle aspiration cytology and core needle biopsy in thyroid nodules: a systematic review and meta-analysis of literature published by Korean authors. Clin Exp Otorhinolaryngol. 2021; 14:116–30.20. Son HM, Kim JH, Kim SC, et al. Distribution and malignancy risk of six categories of the pathology reporting system for thyroid coreneedle biopsy in 1,216 consecutive thyroid nodules. Ultrasonography. 2020; 39:159–65.21. Suh CH, Baek JH, Choi YJ, et al. Efficacy and safety of core-needle biopsy in initially detected thyroid nodules via propensity score analysis. Sci Rep. 2017; 7:8242.22. Ahn HS, Seo M, Ha SM, Kim HS. Comparison of the diagnostic efficacy of ultrasound-guided core needle biopsy with 18- versus 20-gauge needles for thyroid nodules. J Ultrasound Med. 2018; 37:2565–74.23. Choe JY, Kwak Y, Kim M, et al. Utility of a formatted pathologic reporting system in thyroid core needle biopsy: a validation study of 1998 consecutive cases. Clin Endocrinol (Oxf). 2018; 88:96–104.24. Chung SR, Baek JH, Park HS, et al. Ultrasound-pathology discordant nodules on core-needle biopsy: malignancy risk and management strategy. Thyroid. 2017; 27:707–13.25. Ha EJ, Baek JH, Lee JH, et al. Core needle biopsy can minimise the non-diagnostic results and need for diagnostic surgery in patients with calcified thyroid nodules. Eur Radiol. 2014; 24:1403–9.26. Ha EJ, Baek JH, Lee JH, et al. Sonographically suspicious thyroid nodules with initially benign cytologic results: the role of a core needle biopsy. Thyroid. 2013; 23:703–8.27. Joo L, Na DG, Kim JH, Seo H. Comparison of core needle biopsy and repeat fine-needle aspiration in avoiding diagnostic surgery for thyroid nodules initially diagnosed as atypia/follicular lesion of undetermined significance. Korean J Radiol. 2022; 23:280–8.28. Kim YH, Kwon HJ, Kim EK, Kwak JY, Moon HJ, Yoon JH. Applying ultrasound-guided core needle biopsy for diagnosis of thyroid masses: preliminary results from a single institution. J Ultrasound Med. 2015; 34:1801–8.29. Park JY, Yi SY, Baek SH, Lee YH, Kwon HJ, Park HJ. Diagnostic efficacy, performance and safety of side-cut core needle biopsy for thyroid nodules: comparison of automated and semi-automated biopsy needles. Endocrine. 2022; 76:341–8.30. Sung JY, Na DG, Kim KS, et al. Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol. 2012; 22:1564–72.31. Xiong Y, Yan L, Nong L, Zheng Y, Li T. Pathological diagnosis of thyroid nodules based on core needle biopsies: comparative study between core needle biopsies and resected specimens in 578 cases. Diagn Pathol. 2019; 14:10.32. Hong MJ, Na DG, Kim SJ, Kim DS. Role of core needle biopsy as a first-line diagnostic tool for thyroid nodules: a retrospective cohort study. Ultrasonography. 2018; 37:244–53.33. Suh CH, Baek JH, Lee JH, et al. The role of core-needle biopsy in the diagnosis of thyroid malignancy in 4580 patients with 4746 thyroid nodules: a systematic review and meta-analysis. Endocrine. 2016; 54:315–28.34. Agarwal S, Bychkov A, Jung CK. Emerging biomarkers in thyroid practice and research. Cancers (Basel). 2021; 14:204.35. Jung CK, Bychkov A, Kakudo K. Update from the 2022 World Health Organization classification of thyroid tumors: a standardized diagnostic approach. Endocrinol Metab (Seoul). 2022; 37:703–18.36. Burge RA, Hobbs GA. Chapter two - Not all RAS mutations are equal: a detailed review of the functional diversity of RAS hot spot mutations.In : O’Bryan JP, Piazza GA, editors. Advances in Cancer Research. Vol. 153. New York: Academic Press;2022. p. 29–61.37. Saliba M, Katabi N, Dogan S, Xu B, Ghossein RA. NRAS Q61R immunohistochemical staining in thyroid pathology: sensitivity, specificity and utility. Histopathology. 2021; 79:650–60.38. Chang S, Choi YL, Shim HS, Lee GK, Ha SY; Korean Cardiopulmonary Pathology Study Group. Usefulness of BRAF VE1 immunohistochemistry in non-small cell lung cancers: a multi-institutional study by 15 pathologists in Korea. J Pathol Transl Med. 2022; 56:334–41.39. Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol. 2002; 26:41–4.40. Choi YJ, Yun JS, Kim DH. Clinical and ultrasound features of cytology diagnosed follicular neoplasm. Endocr J. 2009; 56:383–9.41. Giorgadze T, Rossi ED, Fadda G, Gupta PK, Livolsi VA, Baloch Z. Does the fine-needle aspiration diagnosis of “Hurthle-cell neoplasm/ follicular neoplasm with oncocytic features” denote increased risk of malignancy? Diagn Cytopathol. 2004; 31:307–12.42. Gulcelik NE, Gulcelik MA, Kuru B. Risk of malignancy in patients with follicular neoplasm: predictive value of clinical and ultrasonographic features. Arch Otolaryngol Head Neck Surg. 2008; 134:1312–5.43. Kim HJ, Mok JO, Kim CH, et al. Preoperative serum thyroglobulin and changes in serum thyroglobulin during TSH suppression independently predict follicular thyroid carcinoma in thyroid nodules with a cytological diagnosis of follicular lesion. Endocr Res. 2017; 42:154–62.44. Kuru B, Kefeli M. Risk factors associated with malignancy and with triage to surgery in thyroid nodules classified as Bethesda category IV (FN/SFN). Diagn Cytopathol. 2018; 46:489–94.45. Lubitz CC, Faquin WC, Yang J, et al. Clinical and cytological features predictive of malignancy in thyroid follicular neoplasms. Thyroid. 2010; 20:25–31.46. Parikh PP, Allan BJ, Lew JI. Surgeon-performed ultrasound predictors of malignancy in patients with Hurthle cell neoplasms of the thyroid. J Surg Res. 2013; 184:247–52.47. Petric R, Perhavec A, Gazic B, Besic N. Preoperative serum thyroglobulin concentration is an independent predictive factor of malignancy in follicular neoplasms of the thyroid gland. J Surg Oncol. 2012; 105:351–6.48. Raber W, Kaserer K, Niederle B, Vierhapper H. Risk factors for malignancy of thyroid nodules initially identified as follicular neoplasia by fine-needle aspiration: results of a prospective study of one hundred twenty patients. Thyroid. 2000; 10:709–12.49. Raparia K, Min SK, Mody DR, Anton R, Amrikachi M. Clinical outcomes for “suspicious” category in thyroid fine-needle aspiration biopsy: patient’s sex and nodule size are possible predictors of malignancy. Arch Pathol Lab Med. 2009; 133:787–90.50. Roth MY, Witt RL, Steward DL. Molecular testing for thyroid nodules: review and current state. Cancer. 2018; 124:888–98.51. Tuttle RM, Lemar H, Burch HB. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid. 1998; 8:377–83.52. Williams MD, Suliburk JW, Staerkel GA, et al. Clinical significance of distinguishing between follicular lesion and follicular neoplasm in thyroid fine-needle aspiration biopsy. Ann Surg Oncol. 2009; 16:3146–53.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surgical Management of Bleeding from the Superior Thyroid Artery after Core Needle Biopsy
- Effectiveness and Limitations of Core Needle Biopsy in the Diagnosis of Thyroid Nodules: Review of Current Literature
- RE: Thyroid Core Needle Biopsy: The Strengths of Guidelines of the Korean Society of Thyroid Radiology
- Thyroid Nodules with Nondiagnostic FNA Results: Role of Core Needle Biopsy
- Diagnostic Performance of Thyroid Core Needle Biopsy Using the Revised Reporting System: Comparison with Fine Needle Aspiration Cytology