Role of Intestinal Microbiota in Metabolism of Voglibose In Vitro and In Vivo
- Affiliations
-
- 0Yeungnam University College of Pharmacy, Gyeongsan, Korea.
- KMID: 2513054
- DOI: http://doi.org/10.4093/dmj.2019.0147
Abstract
Background Voglibose, an α-glucosidase inhibitor, inhibits breakdown of complex carbohydrates into simple sugar units in intestine. Studies showed that voglibose metabolism in the liver might be negligible due to its poor intestinal absorption. Numerous microorganisms live in intestine and have several roles in metabolism and detoxification of various xenobiotics. Due to the limited information, the possible metabolism of voglibose by intestinal microbiota was investigated
in vitro andin vivo .Methods For the
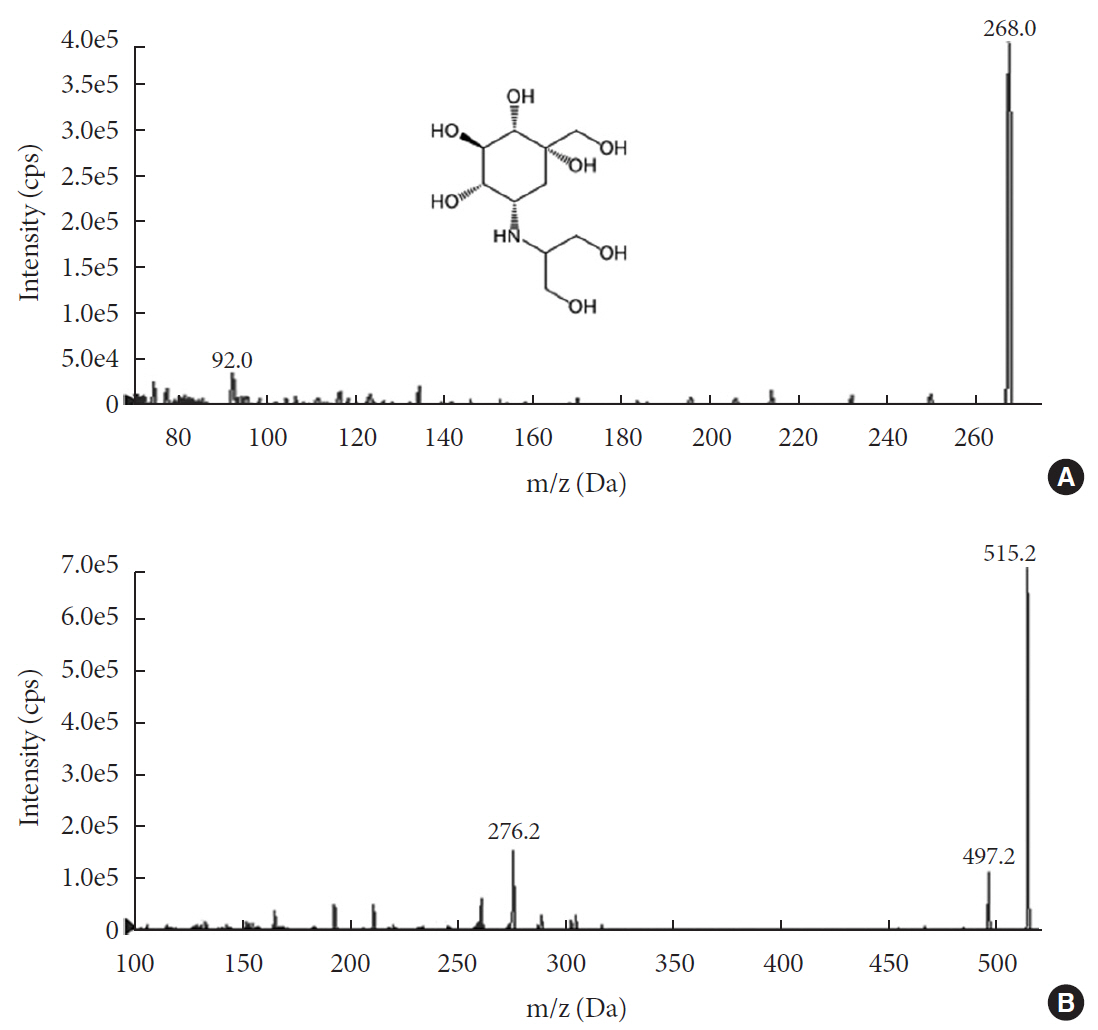
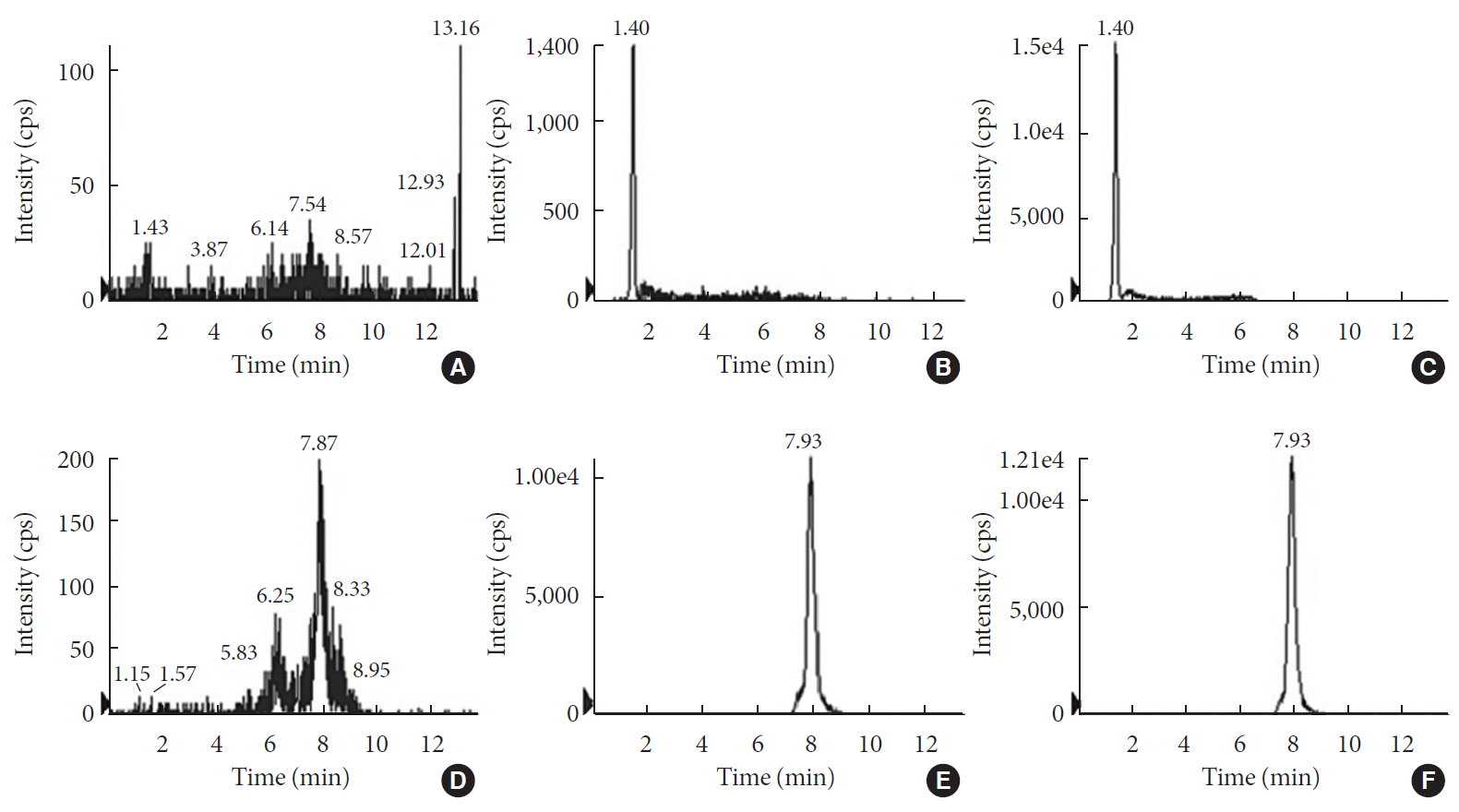
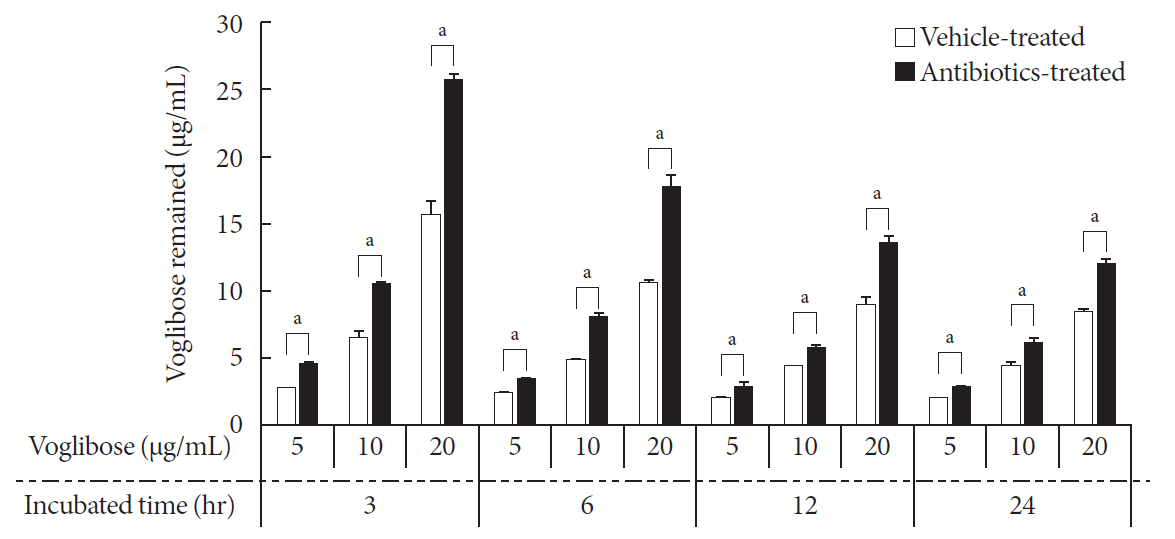
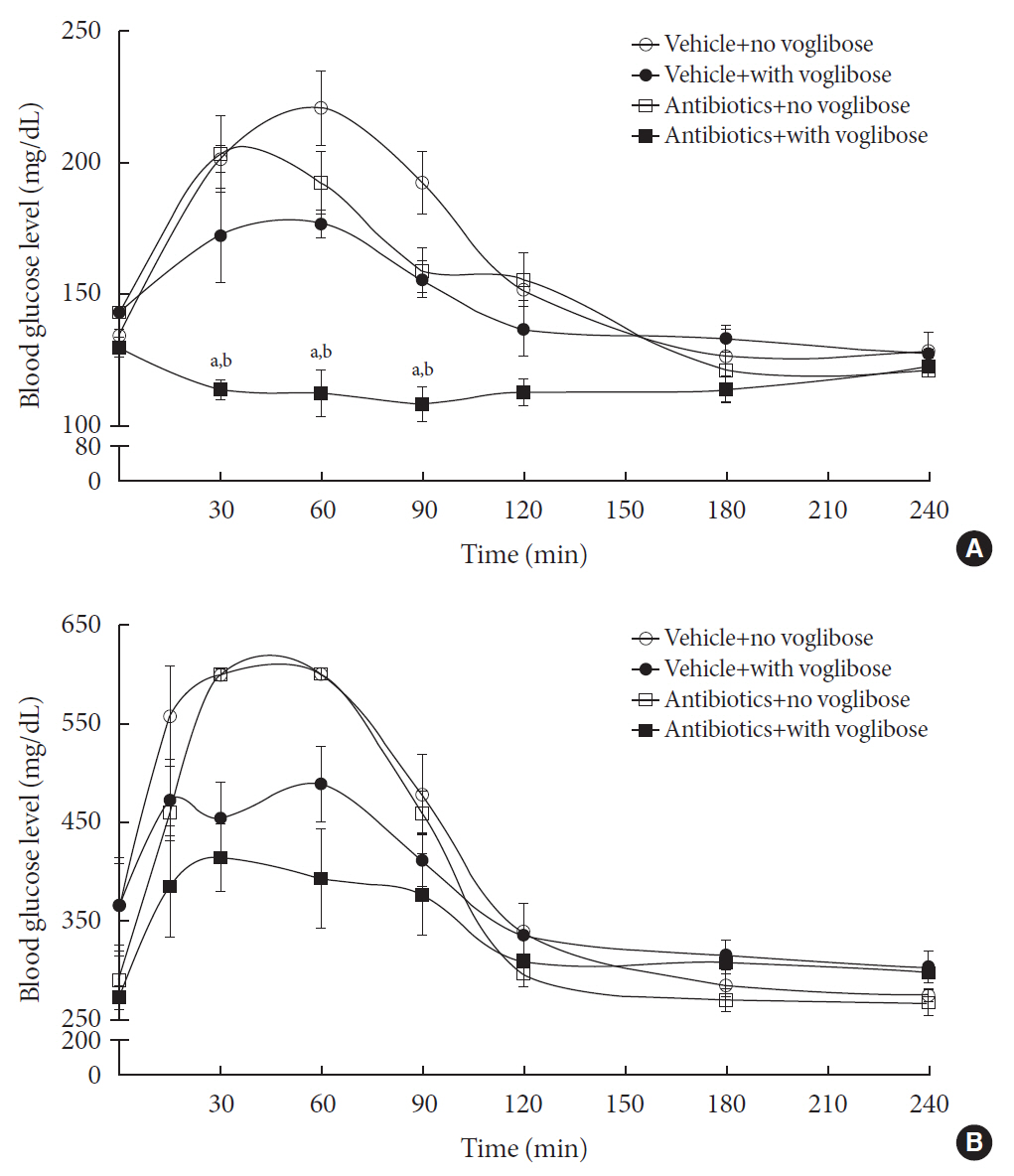
in vitro study, different concentrations of voglibose were incubated with intestinal contents, prepared from both vehicle- and antibiotics-treated mice, to determine the decreased amount of voglibose over time by using liquid chromatography-mass spectrometry. Similarly,in vivo pharmacodynamic effect of voglibose was determined following the administration of voglibose and starch in vehicle- and antibiotic-pretreated non-diabetic and diabetic mice, by measuring the modulatory effects of voglibose on blood glucose levels.Results The
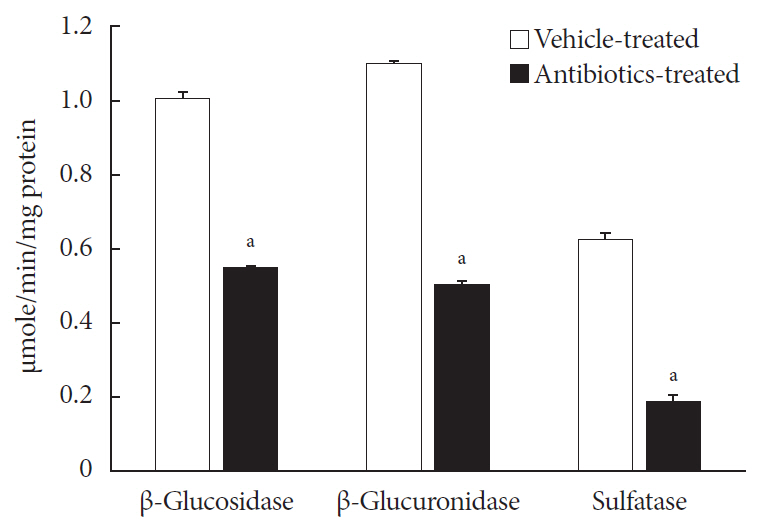
in vitro results indicated that the remaining voglibose could be significantly decreased when incubated with the intestinal contents from normal mice compared to those from antibiotic-treated mice, which had less enzyme activities. Thein vivo results showed that the antibiotic pretreatment resulted in reduced metabolism of voglibose. This significantly lowered blood glucose levels in antibiotic-pretreated mice compared to the control animals.Conclusion The present results indicate that voglibose would be metabolized by the intestinal microbiota, and that this metabolism might be pharmacodynamically critical in lowering blood glucose levels in mice.
Figure
Reference
-
1. O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006; 7:688–693.2. Scarpellini E, Ianiro G, Attili F, Bassanelli C, De Santis A, Gasbarrini A. The human gut microbiota and virome: potential therapeutic implications. Dig Liver Dis. 2015; 47:1007–1012.
Article3. Hur KY, Lee MS. Gut microbiota and metabolic disorders. Diabetes Metab J. 2015; 39:198–203.
Article4. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017; 474:1823–1836.
Article5. Liang D, Leung RK, Guan W, Au WW. Involvement of gut microbiome in human health and disease: brief overview, knowledge gaps and research opportunities. Gut Pathog. 2018; 10:3.
Article6. Dore J, Simren M, Buttle L, Guarner F. Hot topics in gut microbiota. United European Gastroenterol J. 2013; 1:311–318.7. Gavhane YN, Yadav AV. Loss of orally administered drugs in GI tract. Saudi Pharm J. 2012; 20:331–344.
Article8. Kang MJ, Kim HG, Kim JS, Oh DG, Um YJ, Seo CS, Han JW, Cho HJ, Kim GH, Jeong TC, Jeong HG. The effect of gut microbiota on drug metabolism. Expert Opin Drug Metab Toxicol. 2013; 9:1295–1308.
Article9. Pellock SJ, Redinbo MR. Glucuronides in the gut: sugar-driven symbioses between microbe and host. J Biol Chem. 2017; 292:8569–8576.
Article10. Noh K, Kang YR, Nepal MR, Shakya R, Kang MJ, Kang W, Lee S, Jeong HG, Jeong TC. Impact of gut microbiota on drug metabolism: an update for safe and effective use of drugs. Arch Pharm Res. 2017; 40:1345–1355.
Article11. Jeong HG, Kang MJ, Kim HG, Oh DG, Kim JS, Lee SK, Jeong TC. Role of intestinal microflora in xenobiotic-induced toxicity. Mol Nutr Food Res. 2013; 57:84–99.
Article12. Wilson ID, Nicholson JK. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. 2017; 179:204–222.
Article13. Yoo DH, Kim IS, Van Le TK, Jung IH, Yoo HH, Kim DH. Gut microbiota-mediated drug interactions between lovastatin and antibiotics. Drug Metab Dispos. 2014; 42:1508–1513.
Article14. Jiao X, Wang Y, Lin Y, Lang Y, Li E, Zhang X, Zhang Q, Feng Y, Meng X, Li B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J Nutr Biochem. 2019; 64:88–100.
Article15. Dabhi AS, Bhatt NR, Shah MJ. Voglibose: an alpha glucosidase inhibitor. J Clin Diagn Res. 2013; 7:3023–3027.16. Oh TJ, Yu JM, Min KW, Son HS, Lee MK, Yoon KH, Song YD, Park JY, Jeong IK, Cha BS, Kim YS, Baik SH, Kim IJ, Kim DM, Kim SR, Lee KW, Park JH, Lee IK, Park TS, Choi SH, Park SW. Efficacy and safety of voglibose plus metformin in patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Metab J. 2019; 43:276–286.
Article17. Mozes S, Sefcikova Z, Bujnakova D, Racek L. Effect of antibiotic treatment on intestinal microbial and enzymatic development in postnatally overfed obese rats. Obesity (Silver Spring). 2013; 21:1635–1642.18. Food and Drug Administration. Guidance for industry: bioanalytical method validation. Rockville: FDA;2001.19. Kim DH. Gut microbiota-mediated drug-antibiotic interactions. Drug Metab Dispos. 2015; 43:1581–1589.
Article20. Hertz FB, Lobner-Olesen A, Frimodt-Moller N. Antibiotic selection of Escherichia coli sequence type 131 in a mouse intestinal colonization model. Antimicrob Agents Chemother. 2014; 58:6139–6144.21. Kang MJ, Ko GS, Oh DG, Kim JS, Noh K, Kang W, Yoon WK, Kim HC, Jeong HG, Jeong TC. Role of metabolism by intestinal microbiota in pharmacokinetics of oral baicalin. Arch Pharm Res. 2014; 37:371–378.
Article22. Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman HJ. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med. 2011; 61:356–360.23. Wu J, Yan LJ. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab Syndr Obes. 2015; 8:181–188.24. King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012; 166:877–894.
Article25. Van Belle TL, Taylor P, von Herrath MG. Mouse models for type 1 diabetes. Drug Discov Today Dis Models. 2009; 6:41–45.
Article26. Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015; 70:5.
Article27. Deeds MC, Anderson JM, Armstrong AS, Gastineau DA, Hiddinga HJ, Jahangir A, Eberhardt NL, Kudva YC. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011; 45:131–140.
Article28. Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976; 193:415–417.
Article29. Zarrinpar A, Chaix A, Xu ZZ, Chang MW, Marotz CA, Saghatelian A, Knight R, Panda S. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun. 2018; 9:2872.
Article30. Rodrigues RR, Greer RL, Dong X, DSouza KN, Gurung M, Wu JY, Morgun A, Shulzhenko N. Antibiotic-induced alterations in gut microbiota are associated with changes in glucose metabolism in healthy mice. Front Microbiol. 2017; 8:2306.
Article31. Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegard A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab. 2015; 100:3633–3640.
Article32. Fujisaka S, Ussar S, Clish C, Devkota S, Dreyfuss JM, Sakaguchi M, Soto M, Konishi M, Softic S, Altindis E, Li N, Gerber G, Bry L, Kahn CR. Antibiotic effects on gut microbiota and metabolism are host dependent. J Clin Invest. 2016; 126:4430–4443.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gut Microbial Influence and Probiotics on Colorectal Cancer
- Role of the Small Intestinal Microbiota in Gastrointestinal Disorders
- High-fat-diet-modulated Gut Microbiota Promotes Intestinal Carcinogenesis
- Role of Intestinal Microbiota in Inflammatory Bowel Diseases
- Epithelial-microbial diplomacy: escalating border tensions drive inflammation in inflammatory bowel disease