Korean J Physiol Pharmacol.
2021 Mar;25(2):97-109. 10.4196/kjpp.2021.25.2.97.
Paeoniflorin treatment regulates TLR4/NF-κB signaling, reduces cerebral oxidative stress and improves white matter integrity in neonatal hypoxic brain injury
- Affiliations
-
- 1Department of Clinical Nutrition, The First People’s Hospital of Yunnan Province, The Affiliated Hospital of Kunming University of Science and Technology, Kunming 650032, Yunnan, China
- 2Department of Clinical Laboratory, The First Affiliated Hospital of Kunming Medical University,Kunming 650032, Yunnan, China
- 3Yunnan Institute of Laboratory Diagnosis, Kunming 650032, Yunnan, China
- 4Yunnan Key Laboratory of Laboratory Medicine, Kunming 650032, Yunnan, China
- 5School of Stomatology, Kunming Medical University, Kunming 650032, Yunnan, China
- 6Department of Pharmacy, The First People’s Hospital of Yunnan Province, The Affiliated Hospital of Kunming University of Science and Technology, Kunming 650032, Yunnan, China
- KMID: 2512953
- DOI: http://doi.org/10.4196/kjpp.2021.25.2.97
Abstract
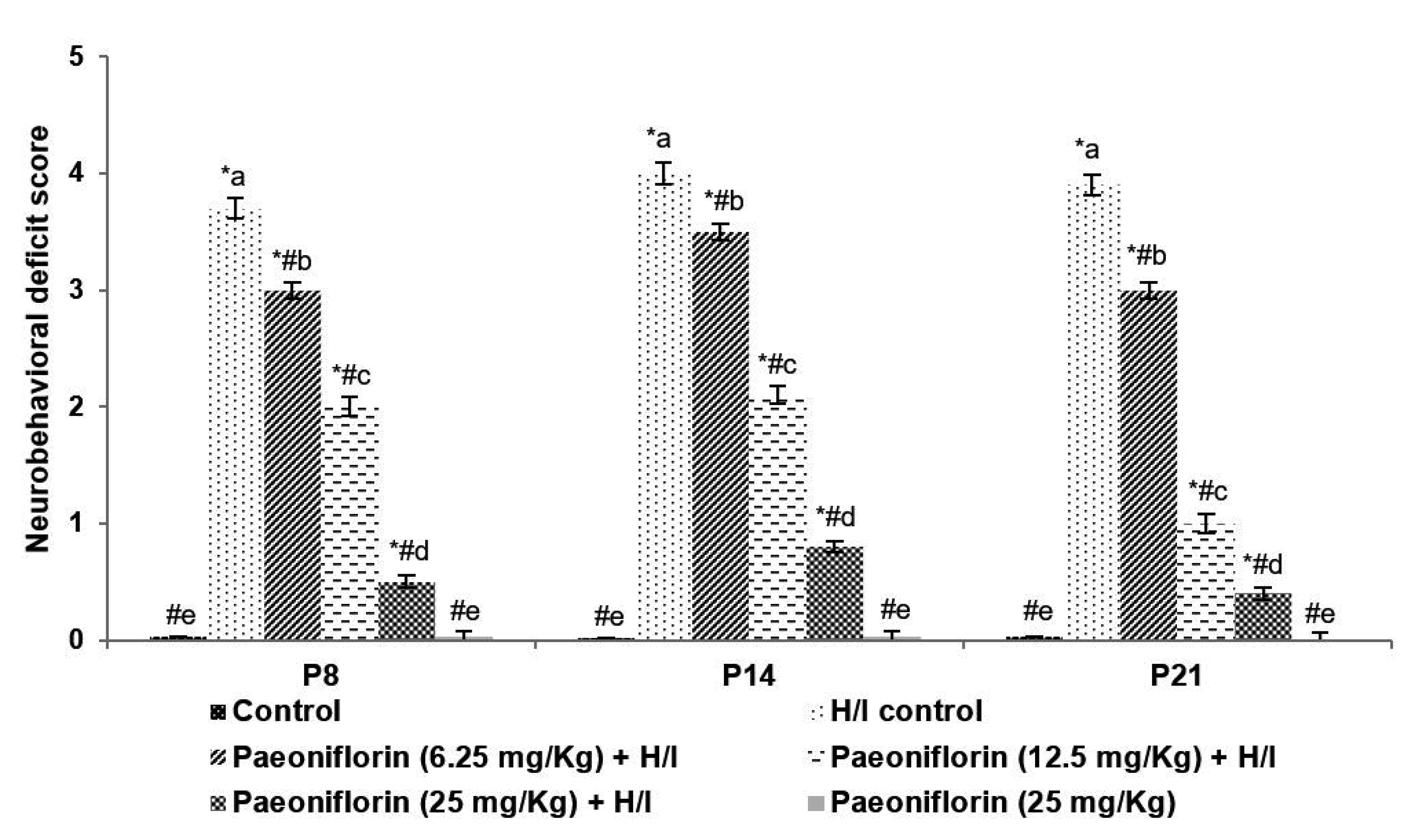
- Neonatal hypoxia/ischemia (H/I), injures white matter, results in neuronal loss, disturbs myelin formation, and neural network development. Neuroinflammation and oxidative stress have been reported in neonatal hypoxic brain injuries. We investigated whether Paeoniflorin treatment reduced H/I-induced inflammation and oxidative stress and improved white matter integrity in a neonatal rodent model. Seven-day old Sprague?Dawley pups were exposed to H/I. Paeoniflorin (6.25, 12.5, or 25 mg/kg body weight) was administered every day via oral gavage from postpartum day 3 (P3) to P14, and an hour before induction of H/I. Pups were sacrificed 24 h (P8) and 72 h (P10) following H/I. Paeoniflorin reduced the apoptosis of neurons and attenuated cerebral infarct volume. Elevated expression of cleaved caspase-3 and Bad were regulated. Paeoniflorin decreased oxidative stress by lowering levels of malondialdehyde and reactive oxygen species generation and while, and it enhanced glutathione content. Microglial activation and the TLR4/NF-κB signaling were significantly down-regulated. The degree of inflammatory mediators (interleukin 1β and tumor necrosis factor-α) were reduced. Paeoniflorin markedly prevented white matter injury via improving expression of myelin binding protein and increasing O1-positive olidgodendrocyte and O4-positive oligodendrocyte counts. The present investigation demonstrates the potent protective efficiency of paeoniflorin supplementation against H/I-induced brain injury by effectually preventing neuronal loss, microglial activation, and white matter injury via reducing oxidative stress and inflammatory pathways.
Figure
Reference
-
1. de Haan M, Wyatt JS, Roth S, Vargha-Khadem F, Gadian D, Mishkin M. 2006; Brain and cognitive-behavioural development after asphyxia at term birth. Dev Sci. 9:350–358. DOI: 10.1111/j.1467-7687.2006.00499.x. PMID: 16764608.
Article2. Hamrick SE, Ferriero DM. 2003; The injury response in the term newborn brain: can we neuroprotect? Curr Opin Neurol. 16:147–154. DOI: 10.1097/01.wco.0000063775.81810.79. PMID: 12644741.
Article3. Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. 2010; Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 340:c363. DOI: 10.1136/bmj.c363. PMID: 20144981. PMCID: PMC2819259.
Article4. Anderson P, Doyle LW. 2003; Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 289:3264–3272. DOI: 10.1001/jama.289.24.3264. PMID: 12824207.
Article5. Hack M, Youngstrom EA, Cartar L, Schluchter M, Taylor HG, Flannery D, Klein N, Borawski E. 2004; Behavioral outcomes and evidence of psychopathology among very low birth weight infants at age 20 years. Pediatrics. 114:932–940. DOI: 10.1542/peds.2003-1017-L. PMID: 15466087.
Article6. Damodaran T, Hassan Z, Navaratnam V, Muzaimi M, Ng G, Müller CP, Liao P, Dringenberg HC. 2014; Time course of motor and cognitive functions after chronic cerebral ischemia in rats. Behav Brain Res. 275:252–258. DOI: 10.1016/j.bbr.2014.09.014. PMID: 25239606.
Article7. Back SA. 2014; Cerebral white and gray matter injury in newborns: new insights into pathophysiology and management. Clin Perinatol. 41:1–24. DOI: 10.1016/j.clp.2013.11.001. PMID: 24524444. PMCID: PMC3947650.8. Huria T, Beeraka NM, Al-Ghamdi B, Fern R. 2015; Premyelinated central axons express neurotoxic NMDA receptors: relevance to early developing white-matter injury. J Cereb Blood Flow Metab. 35:543–553. DOI: 10.1038/jcbfm.2014.227. PMID: 25515212. PMCID: PMC4420873.
Article9. Murray AL, Thompson DK, Pascoe L, Leemans A, Inder TE, Doyle LW, Anderson JFI, Anderson PJ. 2016; White matter abnormalities and impaired attention abilities in children born very preterm. Neuroimage. 124(Pt A):75–84. DOI: 10.1016/j.neuroimage.2015.08.044. PMID: 26318524. PMCID: PMC4791057.
Article10. Song FE, Huang JL, Lin SH, Wang S, Ma GF, Tong XP. 2017; Roles of NG2-glia in ischemic stroke. CNS Neurosci Ther. 23:547–553. DOI: 10.1111/cns.12690. PMID: 28317272. PMCID: PMC6492766.
Article11. Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. 2001; Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 21:1302–1312. DOI: 10.1523/JNEUROSCI.21-04-01302.2001. PMID: 11160401. PMCID: PMC6762224.
Article12. Back SA, Riddle A, McClure MM. 2007; Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 38(2 Suppl):724–730. DOI: 10.1161/01.STR.0000254729.27386.05. PMID: 17261726.
Article13. McQuillen PS, Ferriero DM. 2004; Selective vulnerability in the developing central nervous system. Pediatr Neurol. 30:227–235. DOI: 10.1016/j.pediatrneurol.2003.10.001. PMID: 15087099.
Article14. McLean C, Ferriero D. 2004; Mechanisms of hypoxic-ischemic injury in the term infant. Semin Perinatol. 28:425–432. DOI: 10.1053/j.semperi.2004.10.005. PMID: 15693399.
Article15. Thorburne SK, Juurlink BH. 1996; Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem. 67:1014–1022. DOI: 10.1046/j.1471-4159.1996.67031014.x. PMID: 8752107.
Article16. Kadhim H, Tabarki B, De Prez C, Rona AM, Sébire G. 2002; Interleukin-2 in the pathogenesis of perinatal white matter damage. Neurology. 58:1125–1128. DOI: 10.1212/WNL.58.7.1125. PMID: 11940709.
Article17. Kaur C, Ling EA. 2009; Periventricular white matter damage in the hypoxic neonatal brain: role of microglial cells. Prog Neurobiol. 87:264–280. DOI: 10.1016/j.pneurobio.2009.01.003. PMID: 19428957.
Article18. Cai Z, Lin S, Fan LW, Pang Y, Rhodes PG. 2006; Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience. 137:425–435. DOI: 10.1016/j.neuroscience.2005.09.023. PMID: 16289838.
Article19. Fan LW, Lin S, Pang Y, Rhodes PG, Cai Z. 2006; Minocycline attenuates hypoxia-ischemia-induced neurological dysfunction and brain injury in the juvenile rat. Eur J Neurosci. 24:341–350. DOI: 10.1111/j.1460-9568.2006.04918.x. PMID: 16836639.
Article20. Carty ML, Wixey JA, Colditz PB, Buller KM. 2008; Post-insult minocycline treatment attenuates hypoxia-ischemia-induced neuroinflammation and white matter injury in the neonatal rat: a comparison of two different dose regimens. Int J Dev Neurosci. 26:477–485. DOI: 10.1016/j.ijdevneu.2008.02.005. PMID: 18387771.
Article21. Ock J, Jeong J, Choi WS, Lee WH, Kim SH, Kim IK, Suk K. 2007; Regulation of Toll-like receptor 4 expression and its signaling by hypoxia in cultured microglia. J Neurosci Res. 85:1989–1995. DOI: 10.1002/jnr.21322. PMID: 17461416.
Article22. Kim SY, Choi YJ, Joung SM, Lee BH, Jung YS, Lee JY. 2010; Hypoxic stress up-regulates the expression of Toll-like receptor 4 in macrophages via hypoxia-inducible factor. Immunology. 129:516–524. DOI: 10.1111/j.1365-2567.2009.03203.x. PMID: 20002786. PMCID: PMC2842498.
Article23. Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. 2003; Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 100:8514–8519. DOI: 10.1073/pnas.1432609100. PMID: 12824464. PMCID: PMC166260.
Article24. Kadhim H, Tabarki B, Verellen G, De Prez C, Rona AM, Sébire G. 2001; Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 56:1278–1284. DOI: 10.1212/WNL.56.10.1278. PMID: 11376173.
Article25. Silveira RC, Procianoy RS. 2003; Interleukin-6 and tumor necrosis factor-alpha levels in plasma and cerebrospinal fluid of term newborn infants with hypoxic-ischemic encephalopathy. J Pediatr. 143:625–629. DOI: 10.1067/S0022-3476(03)00531-6. PMID: 14615734.26. Cai Z, Lin S, Pang Y, Rhodes PG. 2004; Brain injury induced by intracerebral injection of interleukin-1beta and tumor necrosis factor-alpha in the neonatal rat. Pediatr Res. 56:377–384. DOI: 10.1203/01.PDR.0000134249.92944.14. PMID: 15201401.
Article27. Liu DF, Wei W, Song LH. 2006; Protective effect of paeoniflorin on immunological liver injury induced by bacillus Calmette-Guerin plus lipopolysaccharide: modulation of tumour necrosis factor-alpha and interleukin-6 MRNA. Clin Exp Pharmacol Physiol. 33:332–339. DOI: 10.1111/j.1440-1681.2006.04371.x. PMID: 16620297.
Article28. Zhong SZ, Ge QH, Li Q, Qu R, Ma SP. 2009; Peoniflorin attentuates Abeta((1-42))-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J Neurol Sci. 280:71–78. DOI: 10.1016/j.jns.2009.01.027. PMID: 19268972.29. Zhou H, Bian D, Jiao X, Wei Z, Zhang H, Xia Y, He Y, Dai Y. 2011; Paeoniflorin protects against lipopolysaccharide-induced acute lung injury in mice by alleviating inflammatory cell infiltration and microvascular permeability. Inflamm Res. 60:981–990. DOI: 10.1007/s00011-011-0359-9. PMID: 21744312.
Article30. Wang Z, Liu Z, Yu G, Nie X, Jia W, Liu RE, Xu R. 2018; Paeoniflorin inhibits migration and invasion of human glioblastoma cells via suppression transforming growth factor β-induced epithelial-mesenchymal transition. Neurochem Res. 43:760–774. DOI: 10.1007/s11064-018-2478-y. PMID: 29423667. PMCID: PMC5842263.
Article31. Garber JC. Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council of the National Academies. Committee for the update of the guide for the care and use of laboratory animals. Guide for the care and use of laboratory animals. 8th ed. 2011. National Academies Press;Washington, D.C.:32. Rice JE 3rd, Vannucci RC, Brierley JB. 1981; The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 9:131–141. DOI: 10.1002/ana.410090206. PMID: 7235629.
Article33. Longa EZ, Weinstein PR, Carlson S, Cummins R. 1989; Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 20:84–91. DOI: 10.1161/01.STR.20.1.84. PMID: 2643202.
Article34. Garcia JH, Wagner S, Liu KF, Hu XJ. 1995; Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 26:627–634. discussion 635DOI: 10.1161/01.STR.26.4.627. PMID: 7709410.35. Arteaga O, Álvarez A, Revuelta M, Santaolalla F, Urtasun A, Hilario E. 2017; Role of antioxidants in neonatal hypoxic-ischemic brain injury: new therapeutic approaches. Int J Mol Sci. 18:265. DOI: 10.3390/ijms18020265. PMID: 28134843. PMCID: PMC5343801.
Article36. Murugan M, Sivakumar V, Lu J, Ling EA, Kaur C. 2011; Expression of N-methyl D-aspartate receptor subunits in amoeboid microglia mediates production of nitric oxide via NF-κB signaling pathway and oligodendrocyte cell death in hypoxic postnatal rats. Glia. 59:521–539. DOI: 10.1002/glia.21121. PMID: 21319220.
Article37. Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. 2008; A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 199:587–595. DOI: 10.1016/j.ajog.2008.06.094. PMID: 19084096.
Article38. Arteaga O, Revuelta M, Urigüen L, Álvarez A, Montalvo H, Hilario E. 2015; Pretreatment with resveratrol prevents neuronal injury and cognitive deficits induced by perinatal hypoxia-ischemia in rats. PLoS One. 10:e0142424. DOI: 10.1371/journal.pone.0142424. PMID: 26544861. PMCID: PMC4636303.
Article39. Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. 2003; Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 85:1026–1036. DOI: 10.1046/j.1471-4159.2003.01756.x. PMID: 12716434.
Article40. Qiu L, Zhu C, Wang X, Xu F, Eriksson PS, Nilsson M, Cooper-Kuhn CM, Kuhn HG, Blomgren K. 2007; Less neurogenesis and inflammation in the immature than in the juvenile brain after cerebral hypoxia-ischemia. J Cereb Blood Flow Metab. 27:785–794. DOI: 10.1038/sj.jcbfm.9600385. PMID: 16926844.
Article41. Wang X, Stridh L, Li W, Dean J, Elmgren A, Gan L, Eriksson K, Hagberg H, Mallard C. 2009; Lipopolysaccharide sensitizes neonatal hypoxic-ischemic brain injury in a MyD88-dependent manner. J Immunol. 183:7471–7477. DOI: 10.4049/jimmunol.0900762. PMID: 19917690.
Article42. Kaur C, You Y. 2000; Ultrastructure and function of the amoeboid microglial cells in the periventricular white matter in postnatal rat brain following a hypoxic exposure. Neurosci Lett. 290:17–20. DOI: 10.1016/S0304-3940(00)01306-9. PMID: 10925164.
Article43. Cunningham C. 2013; Microglia and neurodegeneration: the role of systemic inflammation. Glia. 61:71–90. DOI: 10.1002/glia.22350. PMID: 22674585.
Article44. Fernandez-Lizarbe S, Pascual M, Guerri C. 2009; Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 183:4733–4744. DOI: 10.4049/jimmunol.0803590. PMID: 19752239.
Article45. Semenza GL. 2003; Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 3:721–732. DOI: 10.1038/nrc1187. PMID: 13130303.
Article46. Pugh CW, Ratcliffe PJ. 2003; Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 9:677–684. DOI: 10.1038/nm0603-677. PMID: 12778166.
Article47. Yao L, Kan EM, Lu J, Hao A, Dheen ST, Kaur C, Ling EA. 2013; Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflammation. 10:23. DOI: 10.1186/1742-2094-10-23. PMID: 23388509. PMCID: PMC3575244.
Article48. Li Q, Verma IM. 2002; NF-kappaB regulation in the immune system. Nat Rev Immunol. 2:725–734. DOI: 10.1038/nri910. PMID: 12360211.49. Shen W, Zhang C, Zhang G. 2002; Nuclear factor kappaB activation is mediated by NMDA and non-NMDA receptor and L-type voltage-gated Ca2+ channel following severe global ischemia in rat hippocampus. Brain Res. 933:23–30. DOI: 10.1016/S0006-8993(02)02291-6. PMID: 11929632.50. Bianchi R, Giambanco I, Donato R. 2010; S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol Aging. 31:665–677. DOI: 10.1016/j.neurobiolaging.2008.05.017. PMID: 18599158.51. Back SA, Rosenberg PA. 2014; Pathophysiology of glia in perinatal white matter injury. Glia. 62:1790–1815. DOI: 10.1002/glia.22658. PMID: 24687630. PMCID: PMC4163108.
Article52. Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A, Craig A, Struve J, Sherman LS, Back SA. 2008; Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 63:520–530. DOI: 10.1002/ana.21359. PMID: 18393269. PMCID: PMC3140464.
Article53. Inder TE, Volpe JJ. 2000; Mechanisms of perinatal brain injury. Semin Neonatol. 5:3–16. DOI: 10.1053/siny.1999.0112. PMID: 10802746.
Article54. Wang X, Hagberg H, Zhu C, Jacobsson B, Mallard C. 2007; Effects of intrauterine inflammation on the developing mouse brain. Brain Res. 1144:180–185. DOI: 10.1016/j.brainres.2007.01.083. PMID: 17320062.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Overexpression of miR-146b-5p Ameliorates Neonatal Hypoxic Ischemic Encephalopathy by Inhibiting IRAK1/TRAF6/TAK1/NF-αB Signaling
- Perinatal Hypoxic-lschemic Brain Injury: MR Findings
- Paeoniflorin ameliorates neuropathic pain-induced depression-like behaviors in mice by inhibiting hippocampal neuroinflammation activated via TLR4/NF-kB pathway
- MR findings of brain damage due to perinatal hypoxia
- Resveratrol pretreatment alleviates NLRP3 inflammasomemediated cardiomyocyte pyroptosis by targeting TLR4/MyD88/ NF-κB signaling cascade in coronary microembolization-induced myocardial damage