Quantification of Thioguanine in DNA Using Liquid Chromatography-Tandem Mass Spectrometry for Routine Thiopurine Drug Monitoring in Patients With Pediatric Acute Lymphoblastic Leukemia
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine, Green Cross Laboratories, Yongin, Korea
- 3Department of Laboratory Medicine and Genetics, Samsung Medical Center, Seoul, Korea
- 4Samsung Biomedical Research Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Department of Pediatrics, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 7Department of Clinical Pharmacology and Therapeutics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 8Department of Health Science and Technology, Samsung Advanced Institute of Health Science and Technology, Sungkyunkwan University, Seoul, Korea
- KMID: 2512679
- DOI: http://doi.org/10.3343/alm.2021.41.2.145
Abstract
- Background
We developed an assay to measure DNA-incorporated 6-thioguanine (DNATG) and validated its clinical applicability in Korean pediatric patients with acute lymphoblastic leukemia (ALL) in order to improve individualized thiopurine treatment and reduce the life-threatening cytotoxicity.
Methods
The DNA-TG assay was developed based on liquid chromatography-tandem mass spectrometry, with isotope-labeled TG-d3 and guanine-d3 as internal standards. This method was applied to 257 samples of pediatric ALL patients. The DNA-TG level was compared with erythrocyte TG nucleotide (RBC-TGN) level in relation to the TPMT and NUDT15 genotypes, which affect thiopurine metabolism, using Spearman’s rank test and repeated measure ANOVA.
Results
For DNA-TG quantification, a linearity range of 10.0-5,000.0 fmol TG/µg DNA; bias for accuracy of –10.4% –3.5%; coefficient of variation for intra- and inter-day precision of 3.4% and 5.8% at 80 fmol TG/µg DNA and of 4.9% and 5.3% at 800 fmol TG/µg DNA, respectively; and recovery of 85.7%–116.2% were achieved without matrix effects or carry-over. The median DNA-TG level in the 257 samples was 106.0 fmol TG/µg DNA (interquartile range, 75.8–150.9). There was a strong correlation between DNA-TG and RBC-TGN levels (ρ = 0.68,ρ < 0.0001). The DNA-TG/RBC-TGN ratio was significantly higher in NUDT15 intermediate metabolizers (*1/*2 and *1/*3) than in patients with wildtype alleles (ρ < 0.0001).
Conclusions
This simple and sensitive method for measuring DNA-TG level can improve therapeutic drug monitoring for thiopurine treatment.
Keyword
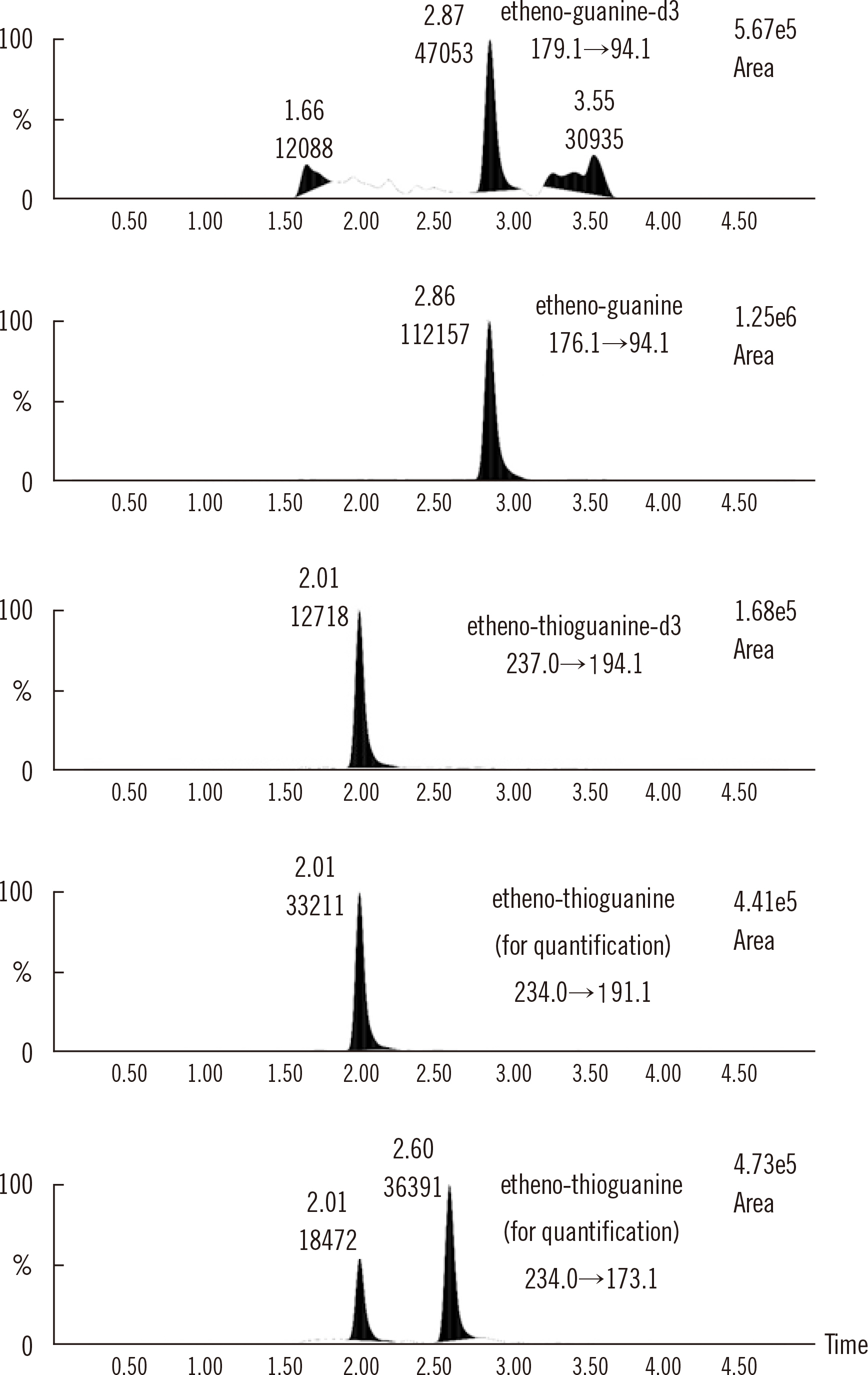
Figure
Cited by 3 articles
-
NUDT15 Genotyping in Thiopurine Drug Therapy
Jong Kwon Lee, Rihwa Choi, Soo-Youn Lee
Lab Med Online. 2022;12(4):217-226. doi: 10.47429/lmo.2022.12.4.217.Comprehensive Evaluation of the NeoBase 2 Non-derivatized MSMS Assay and Exploration of Analytes With Significantly Different Concentrations Between Term and Preterm Neonates
Beomki Lee, Won Young Heo, Jee Ah Kim, Hyun-Seung Lee, Narae Hwang, Hyung-Doo Park, Se In Sung, Yun Sil Chang, Won Soon Park, Soo-Youn Lee
Ann Lab Med. 2023;43(2):153-166. doi: 10.3343/alm.2023.43.2.153.Evaluation of Vancomycin Area Under the Concentration–Time Curve Predictive Performance Using Bayesian Modeling Software With and Without Peak Concentration: An Academic Hospital Experience for Adult Patients Without Renal Impairment
Hyun-Ki Kim, Tae-Dong Jeong
Ann Lab Med. 2023;43(6):554-564. doi: 10.3343/alm.2023.43.6.554.
Reference
-
1. Coulthard SA, Berry P, McGarrity S, Ansari A, Redfern CPF. 2016; Liquid chromatography-mass spectrometry for measuring deoxythioguanosine in DNA from thiopurine-treated patients. J Chromatogr B Analyt Technol Biomed Life Sci. 1028:175–80. DOI: 10.1016/j.jchromb.2016.06.017. PMID: 27362994. PMCID: PMC4955110.
Article2. Moon SY, Lim JH, Kim EH, Nam Y, Yu KS, Hong KT, et al. 2019; Quantification of thiopurine nucleotides in erythrocytes and clinical application to pediatric acute lymphoblastic leukemia. Ther Drug Monit. 41:7585. DOI: 10.1097/FTD.0000000000000575. PMID: 30507626. PMCID: PMC6358190.
Article3. Lampič K, Trontelj J, Prosen H, Drobne D, Šmid A, Vovk T. 2019; Determination of 6-thioguanine and 6-methylmercaptopurine in dried blood spots using liquid chromatography-tandem mass spectrometry: Method development, validation and clinical application. Clin Chim Acta. 499:24–33. DOI: 10.1016/j.cca.2019.08.024. PMID: 31449774.
Article4. Moriyama T, Nishii R, Lin TN, Kihira K, Toyoda H, Jacob N, et al. 2017; The effects of inherited NUDT15 polymorphisms on thiopurine active metabolites in Japanese children with acute lymphoblastic leukemia. Pharmacogenet Genomics. 27:236–9. DOI: 10.1097/FPC.0000000000000282. PMID: 28445187. PMCID: PMC5510236.
Article5. Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, et al. 2016; NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 48:367–73. DOI: 10.1038/ng.3508. PMID: 26878724. PMCID: PMC5029084.6. CLSI. 2014. Liquid chromatography-mass spectrometry methods; approved guideline. CLSI document C62-A. Clinical and Laboratory Standards Institute;Wayne, PA:7. Jacobsen JH, Schmiegelow K, Nersting J. 2012; Liquid chromatography-tandem mass spectrometry quantification of 6-thioguanine in DNA using endogenous guanine as internal standard. J Chromatogr B Analyt Technol Biomed Life Sci. 881-882:115–8. DOI: 10.1016/j.jchromb.2011.11.032. PMID: 22178190.
Article8. Bioanalytical method validation guidance for industry, 2018. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER, Center for Veterinary Medicine (CVM);https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry. Updated on Feb 2020.9. Zaza G, Cheok M, Krynetskaia N, Thorn C, Stocco G, Hebert JM, et al. 2010; Thiopurine pathway. Pharmacogenet Genomics. 20:573–4. DOI: 10.1097/FPC.0b013e328334338f. PMID: 19952870. PMCID: PMC3098750.
Article10. Relling MV, Schwab M, Whirl-Carrillo M, Suarez-Kurtz G, Pui CH, Stein CM, et al. 2019; Clinical Pharmacogenetics Implementation Consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin Pharmacol Ther. 105:1095–105. DOI: 10.1002/cpt.1304. PMID: 30447069. PMCID: PMC6576267.11. Adam de Beaumais T, Fakhoury M, Medard Y, Azougagh S, Zhang D, Yakouben K, et al. 2011; Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol. 71:575–84. DOI: 10.1111/j.1365-2125.2010.03867.x. PMID: 21395650. PMCID: PMC3080646.
Article12. Kim HY, Lee SH, Lee MN, Kim JW, Kim YH, Kim MJ, et al. 2015; Complete sequence-based screening of TPMT variants in the Korean population. Pharmacogenet Genomics. 25:143–6. DOI: 10.1097/FPC.0000000000000117. PMID: 25564374.13. Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. 2015; Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 33:1235–42. DOI: 10.1200/JCO.2014.59.4671. PMID: 25624441. PMCID: PMC4375304.14. Warren DJ, Andersen A, Slørdal L. 1995; Quantitation of 6-thioguanine residues in peripheral blood leukocyte DNA obtained from patients receiving 6-mercaptopurine-based maintenance therapy. Cancer Res. 55:1670–4. PMID: 7712473.15. Nielsen SN, Grell K, Nersting J, Frandsen TL, Hjalgrim LL, Schmiegelow K. 2016; Measures of 6-mercaptopurine and methotrexate maintenance therapy intensity in childhood acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 78:983–94. DOI: 10.1007/s00280-016-3151-2. PMID: 27600880.
Article16. Nielsen SN, Grell K, Nersting J, Abrahamsson J, Lund B, Kanerva J, et al. 2017; DNA-thioguanine nucleotide concentration and relapse-free survival during maintenance therapy of childhood acute lymphoblastic leukaemia (NOPHO ALL2008): a prospective substudy of a phase 3 trial. Lancet Oncol. 18:515–24. DOI: 10.1016/S1470-2045(17)30154-7. PMID: 28258828.
Article17. Draft guideline on bioanalytical method validation in pharmaceutical development. 2013. Ministry of Health and Welfare;Japan: http://www.nihs.go.jp/drug/BMV/BMV_draft_130415_E.pdf. Updated on Feb 2020.18. Guideline on bioanalytical. European Medicine Agency;2012. https://www.ema.europa.eu/en/bioanalytical-method-validation. Updated on Feb 2020.19. CLSI. 2007. Mass spectrometry in the clinical laboratory: general principles and guidance; approved guideline. CLSI document C50-A. Clinical and Laboratory Standards Institute;Wayne, PA:20. Scientific Working Group for Forensic Toxicology. 2013; Scientific Working Group for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology. J Anal Toxicol. 37:452–74. DOI: 10.1093/jat/bkt054. PMID: 23934984.21. Guideline on bioanalytical method validation, 2013. 2013. Ministry of Food and Drug Safety;Republic of Korea: https://www.mfds.go.kr/brd/m_210/view.do?seq=13054. Updated on Feb 2020.22. Dervieux T, Meyer G, Barham R, Matsutani M, Barry M, Boulieu R, et al. 2005; Liquid chromatography-tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effect of thiopurine methyltransferase gene variants on these metabolites in patients receiving azathioprine/6mercaptopurine therapy. Clin Chem. 51:2074–84. DOI: 10.1373/clinchem.2005.050831. PMID: 16166171.
Article23. Lee MN, Kang B, Choi SY, Kim MJ, Woo SY, Kim JW, et al. 2015; Impact of genetic polymorphisms on 6-thioguanine nucleotide levels and toxicity in pediatric patients with IBD treated with azathioprine. Inflamm Bowel Dis. 21:2897–908. DOI: 10.1097/MIB.0000000000000570. PMID: 26332308.
Article24. Yoo IY, Lee K, Ji OJ, Woo HI, Lee SY. 2018; Evaluation of stability of thiopurine metabolites using a validated LC-MS/MS method. Ann Lab Med. 38:255–60. DOI: 10.3343/alm.2018.38.3.255. PMID: 29401561. PMCID: PMC5820071.
Article25. Kim HT, Choi R, Won HH, Choe YH, Kang B, Lee K, et al. 2017; NUDT15 genotype distributions in the Korean population. Pharmacogenet Genomics. 27:197–200. DOI: 10.1097/FPC.0000000000000274. PMID: 28277331.26. Coulthard SA, Hogarth LA, Little M, Matheson EC, Redfern CP, Minto L, et al. 2002; The effect of thiopurine methyltransferase expression on sensitivity to thiopurine drugs. Mol Pharmacol. 62:102–9. DOI: 10.1124/mol.62.1.102. PMID: 12065760.
Article27. Choi R, Sohn I, Kim MJ, Woo HI, Lee JW, Ma Y, et al. 2019; Pathway genes and metabolites in thiopurine therapy in Korean children with acute lymphoblastic leukaemia. Br J Clin Pharmacol. 85:1585–97. DOI: 10.1111/bcp.13943. PMID: 30927276. PMCID: PMC6595296.
Article28. Yang JJ, Whirl-Carrillo M, Scott SA, Turner AJ, Schwab M, Tanaka Y, et al. 2019; Pharmacogene Variation Consortium gene introduction: NUDT15. Clin Pharmacol Ther. 105:1091. DOI: 10.1002/cpt.1268. PMID: 30515762.29. Selinger CP, Ochieng AO, George V, Leong RW. 2019; The accuracy of adherence self-report scales in patients on thiopurines for inflammatory bowel disease: a comparison with drug metabolite levels and medication possession ratios. Inflamm Bowel Dis. 25:919–924. DOI: 10.1093/ibd/izy309. PMID: 30265299.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Stability of Thiopurine Metabolites Using a Validated LC-MS/MS Method
- Pancreatitis Induced by 6-mercaptopurine and 6-thioguanine in Childhood Acute Lymphoblastic Leukemia
- Development and validation of analytical method for the determination of radotinib in human plasma using liquid chromatography-tandem mass spectrometry
- Multiplex Assay of Second-Line Anti-Tuberculosis Drugs in Dried Blood Spots Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry
- A Liquid Chromatography-Tandem Mass Spectrometry Method for Simultaneously Determining Meropenem and Linezolid in Blood and Cerebrospinal Fluid