Ann Lab Med.
2018 May;38(3):255-260. 10.3343/alm.2018.38.3.255.
Evaluation of Stability of Thiopurine Metabolites Using a Validated LC-MS/MS Method
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. suddenbz@skku.edu
- 2Department of Laboratory Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- KMID: 2403460
- DOI: http://doi.org/10.3343/alm.2018.38.3.255
Abstract
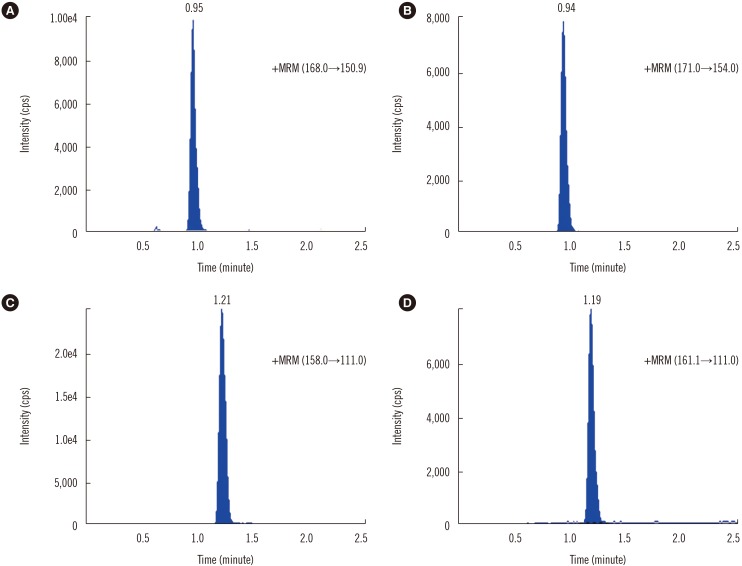
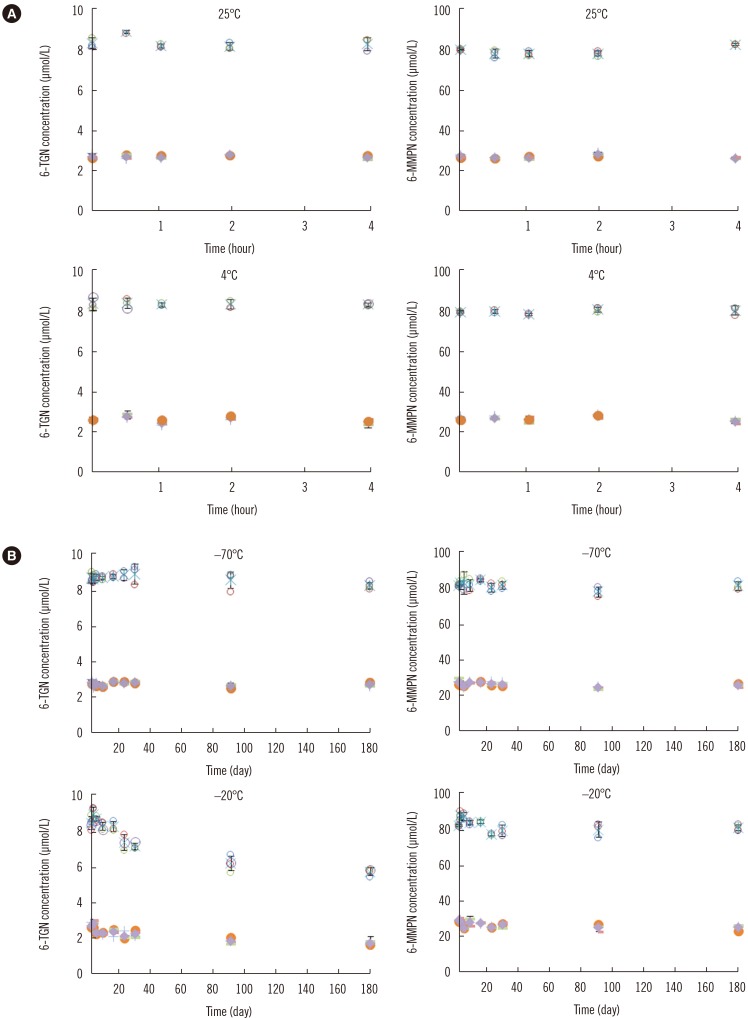
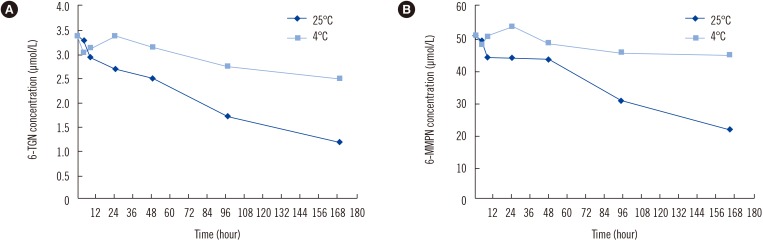
- Measurement of thiopurine metabolites is helpful to monitor adverse effects and assess compliance in patients on thiopurine treatment. The purpose of this study was to develop and validate an analytical method for measurement of thiopurine metabolites, thioguanine nucleotides (6-TGN) and 6-methylmercaptopurine nucleotide (6-MMPN), in RBCs. We developed and validated a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the quantification of 6-TGN and 6-MMPN and evaluated the stability of the thiopurine metabolites in RBC and whole blood states without any preprocessing at various storage conditions. The linear range was 0.1-10 µmol/L and 0.5-100 µmol/L for 6-TGN and 6-MMPN, respectively. The mean extraction recovery at the two concentrations was 71.0% and 75.0% for 6-TGN, and 102.2% and 96.4% for 6-MMPN. Thiopurine metabolites in preprocessed RBC samples were stable at 25℃ and 4℃ after storage for 4 hours and at −70℃ for up to 6 months. However, 6-TGN decreased by 30% compared with the initial concentration when stored at −20℃ for 180 days. In whole blood states, 6-TGN decreased by about 20% at four days after storage at 4℃. We validated a reliable LC-MS/MS method and recommend that the patient's whole blood sample be preprocessed as soon as possible.
Keyword
Figure
Cited by 2 articles
-
Quantification of Thioguanine in DNA Using Liquid Chromatography-Tandem Mass Spectrometry for Routine Thiopurine Drug Monitoring in Patients With Pediatric Acute Lymphoblastic Leukemia
Rihwa Choi, Mi Ryung Chun, Jisook Park, Ji Won Lee, Hee Young Ju, Hee Won Cho, Ju Kyung Hyun, Hong Hoe Koo, Eun Sang Yi, Soo-Youn Lee
Ann Lab Med. 2021;41(2):145-154. doi: 10.3343/alm.2021.41.2.145.Review of the Use of Liquid Chromatography-Tandem Mass Spectrometry in Clinical Laboratories: Part I-Development
Brian A. Rappold
Ann Lab Med. 2022;42(2):121-140. doi: 10.3343/alm.2022.42.2.121.
Reference
-
1. Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer. 2008; 8:24–36. PMID: 18097462.2. Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol. 2008; 64:753–767. PMID: 18506437.3. Gearry RB, Barclay ML. Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J Gastroenterol Hepatol. 2005; 20:1149–1157. PMID: 16048561.4. Lee MN, Kang B, Choi SY, Kim MJ, Woo SY, Kim JW, et al. Impact of genetic polymorphisms on 6-thioguanine nucleotide levels and toxicity in pediatric patients with IBD treated with azathioprine. Inflamm Bowel Dis. 2015; 21:2897–2908. PMID: 26332308.5. Dervieux T, Boulieu R. Simultaneous determination of 6-thioguanine and methyl 6-mercaptopurine nucleotides of azathioprine in red blood cells by HPLC. Clin Chem. 1998; 44:551–555. PMID: 9510860.6. Vikingsson S, Almer S, Peterson C, Carlsson B, Josefsson M. Monitoring of thiopurine metabolites – a high-performance liquid chromatography method for clinical use. J Pharm Biomed Anal. 2013; 75:145–152. PMID: 23261807.7. Nygaard U, Toft N, Schmiegelow K. Methylated metabolites of 6-mercaptopurine are associated with hepatotoxicity. Clin Pharmacol Ther. 2004; 75:274–281. PMID: 15060506.8. Dubinsky MC, Yang H, Hassard PV, Seidman EG, Kam LY, Abreu MT, et al. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology. 2002; 122:904–915. PMID: 11910342.9. Cuffari C, Dassopoulos T, Turnbough L, Thompson RE, Bayless TM. Thiopurine methyltransferase activity influences clinical response to azathioprine in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2004; 2:410–417. PMID: 15118980.10. Dervieux T, Meyer G, Barham R, Matsutani M, Barry M, Boulieu R, et al. Liquid chromatography-tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effect of thiopurine methyltransferase gene variants on these metabolites in patients receiving azathioprine/6-mercaptopurine therapy. Clin Chem. 2005; 51:2074–2084. PMID: 16166171.11. Cangemi G, Barabino A, Barco S, Parodi A, Arrigo S, Melioli G. A validated HPLC method for the monitoring of thiopurine metabolites in whole blood in paediatric patients with inflammatory bowel disease. Int J Immunopathol Pharmacol. 2012; 25:435–444. PMID: 22697075.12. Neurath MF, Kiesslich R, Teichgraber U, Fischer C, Hofmann U, Eichelbaum M, et al. 6-thioguanosine diphosphate and triphosphate levels in red blood cells and response to azathioprine therapy in Crohn’s disease. Clin Gastroenterol Hepatol. 2005; 3:1007–1014. PMID: 16234047.13. de Graaf P, de Boer NK, Jharap B, Mulder CJ, van Bodegraven AA, Veldkamp AI. Stability of thiopurine metabolites: a potential analytical bias. Clin Chem. 2008; 54:216–218. PMID: 18160731.14. Pike MG, Franklin CL, Mays DC, Lipsky JJ, Lowry PW, Sandborn WJ. Improved methods for determining the concentration of 6-thioguanine nucleotides and 6-methylmercaptopurine nucleotides in blood. J Chromatogr B Biomed Sci Appl. 2001; 757:1–9. PMID: 11419732.15. Kirchherr H, Shipkova M, von Ahsen N. Improved method for therapeutic drug monitoring of 6-thioguanine nucleotides and 6-methylmercaptopurine in whole-blood by LC/MSMS using isotope-labeled internal standards. Ther Drug Monit. 2013; 35:313–321. PMID: 23666567.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of MassTrak LC/MS/MS Tacrolimus Kit
- The Stability Evaluation of Plasma and Urine Metanephrines for Diagnosing Pheochromocytoma and Paraganglioma by LC-MS/MS
- Determination of Phthalate Metabolites in Human Serum and Urine as Biomarkers for Phthalate Exposure Using Column-Switching LC-MS/MS
- An Accurate Isotope Dilution Liquid Chromatography-Tandem Mass Spectrometry Method for Serum C-Peptide and Its Use in Harmonization in China
- LC–MS/MS-Based Comparative Investigation on Chemical Constituents of Six Aster Species Occurring in Korea