Ann Lab Med.
2016 Sep;36(5):489-493. 10.3343/alm.2016.36.5.489.
Multiplex Assay of Second-Line Anti-Tuberculosis Drugs in Dried Blood Spots Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea. songjhcp@snu.ac.kr
- 2Department of Laboratory Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Laboratory Medicine, Sheikh Khalifa Specialty Hospital, Ras Al Khaimah, United Arab Emirates.
- 5Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2373581
- DOI: http://doi.org/10.3343/alm.2016.36.5.489
Abstract
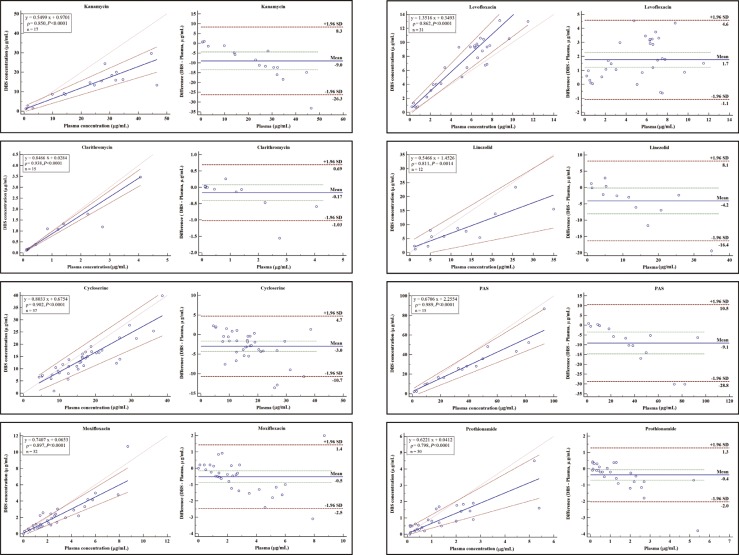
- As dried blood spots (DBSs) have various advantages over conventional venous blood sampling, some assays for detection of one or two anti-tuberculosis (TB) drugs in DBSs have been developed. However, there are no assays currently available for the simultaneous measurement of three or more anti-TB drugs in DBSs. In this study, we developed and evaluated a multiplex method for detecting nine anti-TB drugs including streptomycin, kanamycin, clarithromycin, cycloserine, moxifloxacin, levofloxacin, para-aminosalicylic acid, prothionamide, and linezolid in DBSs by using ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Seventy-nine patient samples of DBS were analyzed on the UPLC-MS/MS system. All drug concentrations were determined within 4 min, and assay performance was evaluated. All drugs were clearly separated without ion suppression. Within-run and between-run precisions were 1.7-13.0% and 5.7-17.0%, respectively, at concentrations representing low and high levels for the nine drugs. Lower limits of detection and quantification were 0.06-0.6 and 0.5-5.0 µg/mL, respectively. Linearity was acceptable at five level concentrations for each drug. Correlations between drug concentrations in plasma and DBSs by using Passing-Bablock regression and Pearson's rho (Ï, 0.798-0.989) were acceptable. In conclusion, we developed a multiplex assay to measure nine second-line anti-TB drugs in DBSs successfully. This assay provided convenient and rapid drug quantification and could have applications in drug monitoring during treatment.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Recommendations for the Use of Liquid Chromatography-Mass Spectrometry in the Clinical Laboratory: Part I. Implementation and Management
Kyunghoon Lee, Soo Young Moon, Serim Kim, Hyun-Jung Choi, Sang-Guk Lee, Hyung-Doo Park, Soo-Youn Lee, Sang Hoon Song,
Lab Med Online. 2020;10(1):1-9. doi: 10.3343/lmo.2020.10.1.1.
Reference
-
1. Sotgiu G, Alffenaar JW, Centis R, D'Ambrosio L, Spanevello A, Piana A, et al. Therapeutic drug monitoring: how to improve drug dosage and patient safety in tuberculosis treatment. Int J Infect Dis. 2015; 32:101–104. PMID: 25809764.2. Park JS, Lee JY, Lee YJ, Kim SJ, Cho YJ, Yoon HI, et al. Serum levels of antituberculosis drugs and their effect on tuberculosis treatment outcome. Antimicrob Agents Chemother. 2015; 60:92–98. PMID: 26459901.3. Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis. 2011; 204:1951–1959. PMID: 22021624.4. Pasipanodya JG, Srivastava S, Gumbo T. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin Infect Dis. 2012; 55:169–177. PMID: 22467670.5. Han M, Jun SH, Lee JH, Park KU, Song J, Song SH. Method for simultaneous analysis of nine second-line anti-tuberculosis drugs using UPLC-MS/MS. J Antimicrob Chemother. 2013; 68:2066–2073. PMID: 23657802.6. Vu DH, Alffenaar JW, Edelbroek PM, Brouwers JR, Uges DR. Dried blood spots: a new tool for tuberculosis treatment optimization. Curr Pharm Des. 2011; 17:2931–2939. PMID: 21834763.7. Vu DH, Koster RA, Alffenaar JW, Brouwers JR, Uges DR. Determination of moxifloxacin in dried blood spots using LC-MS/MS and the impact of the hematocrit and blood volume. J Chromatogr B Analyt Technol Biomed Life Sci. 2011; 879:1063–1070.8. Vu DH, Bolhuis MS, Koster RA, Greijdanus B, de Lange WC, van Altena R, et al. Dried blood spot analysis for therapeutic drug monitoring of linezolid in patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2012; 56:5758–5763. PMID: 22926568.9. Vu DH, Koster RA, Bolhuis MS, Greijdanus B, Altena RV, Nguyen DH, et al. Simultaneous determination of rifampicin, clarithromycin and their metabolites in dried blood spots using LC-MS/MS. Talanta. 2014; 121:9–17. PMID: 24607103.10. Kim B, Lee MN, Park HD, Kim JW, Chang YS, Park WS, et al. Dried blood spot testing for seven steroids using liquid chromatography-tandem mass spectrometry with reference interval determination in the Korean population. Ann Lab Med. 2015; 35:578–585. PMID: 26354345.11. Legnini E, Orsini JJ, Mühl A, Johnson B, Dajnoki A, Bodamer OA. Analysis of acid sphingomyelinase activity in dried blood spots using tandem mass spectrometry. Ann Lab Med. 2012; 32:319–323. PMID: 22950066.12. Annesley TM. Ion suppression in mass spectrometry. Clin Chem. 2003; 49:1041–1044. PMID: 12816898.13. Clinical Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. CLSI Document EP6-A. Wayne, PA: Clinical Laboratory Standards Institute;2003.14. Park SI, Oh J, Jang K, Yoon J, Moon SJ, Park JS, et al. Pharmacokinetics of second-line antituberculosis drugs after multiple administrations in healthy volunteers. Antimicrob Agents Chemother. 2015; 59:4429–4435. PMID: 25987620.15. Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012; 24:69–71. PMID: 23638278.16. Capiau S, Stove VV, Lambert WE, Stove CP. Prediction of the hematocrit of dried blood spots via potassium measurement on a routine clinical chemistry analyzer. Anal Chem. 2013; 85:404–410. PMID: 23190205.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Simultaneous Screening of 177 Drugs of Abuse in Urine Using Ultra-performance Liquid Chromatography with Tandem Mass Spectrometry in Drug-intoxicated Patients
- Bioanalytical methods for the detection of duloxetine and thioctic acid in plasma using ultra performance liquid chromatography with tandem mass spectrometry (UPLC-MS/MS)
- Evaluation of the Triage TOX Drug Screen Assay for Detection of 11 Drugs of Abuse and Therapeutic Drugs
- Use of Tandem Mass Spectrometry for Newborn Screening of 6 Lysosomal Storage Disorders in a Korean Population
- Measurement of Serum Levels of 25-Hydroxyvitamin D3 and 25-Hydroxyvitamin D2 Using Diels-Alder Derivatization and Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry