J Korean Med Sci.
2020 Dec;35(50):e427. 10.3346/jkms.2020.35.e427.
Cannabidiol for Treating LennoxGastaut Syndrome and Dravet Syndrome in Korea
- Affiliations
-
- 1Division of Pediatric Emergency, Department of Pediatrics, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Division of Pediatric Neurology, Department of Pediatrics, Epilepsy Research Institute, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea
- 4National Academy of Medicine of Korea, Seoul, Korea
- 5Department of Psychiatry, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2509756
- DOI: http://doi.org/10.3346/jkms.2020.35.e427
Abstract
- Background
For the first time in Korea, we aimed to study the efficacy and safety of cannabidiol (CBD), which is emerging as a new alternative in treating epileptic encephalopathies.
Methods
This study was conducted retrospectively with patients between the ages of 2–18 years diagnosed with Lennox-Gastaut syndrome (LGS) or Dravet syndrome (DS) were enrolled from March to October 2019, who visited outpatient unit at 3 and 6 months to evaluate medication efficacy and safety based on caregiver reporting. Additional evaluations, such as electroencephalogram and blood tests, were conducted at each period also. CBD was administered orally at a starting dose of 5 mg/kg/day, and was maintained at 10 mg/kg/day.
Results
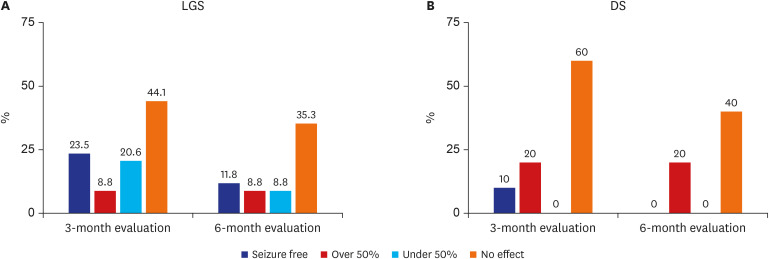
We analyzed 34 patients in the LGS group and 10 patients in the DS group between the ages of 1.2–15.8 years. In the 3-month evaluation, the overall reduction of seizure frequency in the LGS group was 52.9% (>50% reduction in 32.3% of the cases), and 29.4% in the 6-month evaluation (more than 50% reduction in 20.6%). In DS group, the reduction of seizure frequency by more than 50% was 30% and 20% in the 3-month and 6-month evaluation, respectively. Good outcomes were defined as the reduction of seizure frequency by more than 50% and similar results were observed in both LGS and DS groups. Adverse events were reported in 36.3% of total patients of which most common adverse events were gastrointestinal problems. However, no life-threatening adverse event was reported in both LGS and DS during the observation period.
Conclusion
In this first Korean study, CBD was safe and tolerable for use and could be expected to potentially reduce the seizure frequency in pediatric patients with LGS or DS.
Figure
Cited by 1 articles
-
Elevated Circulating Sclerostin Levels in Frail Older Adults: Implications beyond Bone Health
Ji Yeon Baek, Seong Hee Ahn, Il-Young Jang, Hee-Won Jung, Eunhye Ji, So Jeong Park, Yunju Jo, Eunju Lee, Dongryeol Ryu, Seongbin Hong, Beom-Jun Kim
Endocrinol Metab. 2025;40(1):73-81. doi: 10.3803/EnM.2024.2100.
Reference
-
1. Donner EJ. Opportunity gained, opportunity lost: treating pharmacoresistant epilepsy in children. Epilepsia. 2013; 54(Suppl 2):16–18. PMID: 23646965.
Article2. Cilio MR, Thiele EA, Devinsky O. The case for assessing cannabidiol in epilepsy. Epilepsia. 2014; 55(6):787–790. PMID: 24854434.
Article3. Berg AT, Zelko FA, Levy SR, Testa FM. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes: a prospective cohort study. Neurology. 2012; 79(13):1384–1391. PMID: 22972641.
Article4. O'Connell BK, Gloss D, Devinsky O. Cannabinoids in treatment-resistant epilepsy: a review. Epilepsy Behav. 2017; 70(Pt B):341–348. PMID: 28188044.5. Koo CM, Kang HC. Could cannabidiol be a treatment option for intractable childhood and adolescent epilepsy? J Epilepsy Res. 2017; 7(1):16–20. PMID: 28775950.
Article6. Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010; 51(4):676–685. PMID: 20196795.
Article7. Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016; 15(3):270–278. PMID: 26724101.
Article8. Klotz KA, Grob D, Hirsch M, Metternich B, Schulze-Bonhage A, Jacobs J. Efficacy and tolerance of synthetic cannabidiol for treatment of drug resistant epilepsy. Front Neurol. 2019; 10:1313. PMID: 31920934.
Article9. Hussain SA, Zhou R, Jacobson C, Weng J, Cheng E, Lay J, et al. Perceived efficacy of cannabidiol-enriched cannabis extracts for treatment of pediatric epilepsy: a potential role for infantile spasms and Lennox-Gastaut syndrome. Epilepsy Behav. 2015; 47:138–141. PMID: 25935511.
Article10. Mitelpunkt A, Kramer U, Hausman Kedem M, Zilbershot Fink E, Orbach R, Chernuha V, et al. The safety, tolerability, and effectiveness of PTL-101, an oral cannabidiol formulation, in pediatric intractable epilepsy: a phase II, open-label, single-center study. Epilepsy Behav. 2019; 98(Pt A):233–237. PMID: 31394352.
Article11. Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018; 391(10125):1085–1096. PMID: 29395273.
Article12. Devinsky O, Cross JH, Wright S. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017; 377(7):699–700.
Article13. Lutz B. On-demand activation of the endocannabinoid system in the control of neuronal excitability and epileptiform seizures. Biochem Pharmacol. 2004; 68(9):1691–1698. PMID: 15450934.
Article14. Pertwee RG, Cascio MG. Known pharmacological actions of delta-9-tetrahydrocannabinol and of four other chemical constituents of cannabis that activate cannabinoid receptors. In : Pertwee RG, editor. Handbook of Cannabis. Oxford: Oxford University Press;2014.15. Bureau M, Genton P, Dravet C, Delgado-Escueta AV, Tassinari CA, Thomas P, et al. Epileptic Syndromes in Infancy, Childhood and Adolescence. 5th ed. Montrouge: John Libbey Eurotext;2012.16. Kane N, Acharya J, Benickzy S, Caboclo L, Finnigan S, Kaplan PW, et al. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin Neurophysiol Pract. 2017; 2:170–185. PMID: 30214992.
Article17. Goldenholz DM, Moss R, Scott J, Auh S, Theodore WH. Confusing placebo effect with natural history in epilepsy: a big data approach. Ann Neurol. 2015; 78(3):329–336. PMID: 26150090.
Article18. Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018; 378(20):1888–1897. PMID: 29768152.
Article19. Miller I, Scheffer IE, Gunning B, Sanchez-Carpintero R, Gil-Nagel A, Perry MS, et al. Dose-ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: a randomized clinical trial. JAMA Neurol. 2020; 77(5):613–621. PMID: 32119035.20. Szaflarski JP, Bebin EM, Comi AM, Patel AD, Joshi C, Checketts D, et al. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: expanded access program results. Epilepsia. 2018; 59(8):1540–1548. PMID: 29998598.
Article21. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014; 46(1):86–95. PMID: 24160757.
Article22. Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: general features and interactions between antiepileptic drugs. Lancet Neurol. 2003; 2(6):347–356. PMID: 12849151.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Cannabidiol on Adaptive Behavior and Quality of Life in Pediatric Patients With Treatment-Resistant Epilepsy
- Current Pharmacologic Strategies for Treatment of Intractable Epilepsy in Children
- SCN1A Variants in Patients with Dravet Syndrome
- The Genetic Facets of Dravet Syndrome: Recent Insights
- SCN1A Gene Mutation and Adaptive Functioning in 18 Vietnamese Children with Dravet Syndrome