Korean J Physiol Pharmacol.
2020 Nov;24(6):453-462. 10.4196/kjpp.2020.24.6.453.
Anti-arthritic activity of D-carvone against complete Freund’s adjuvant-induced arthritis in rats through modulation of inflammatory cytokines
- Affiliations
-
- 1Rheumatism and Immunology Ward of Integrated Traditional Chinese and Western Medicine, China
- 2Department of Dermatology Pain Management, Central Hospital Affiliated to Shandong First Medical University, Jinan, Shandong Province 250013, P. R. China
- 3Department of Rheumatology and Immunology, Central Hospital Affiliated to Shandong First Medical University, Jinan, Shandong Province 250013, P. R. China
- KMID: 2507728
- DOI: http://doi.org/10.4196/kjpp.2020.24.6.453
Abstract
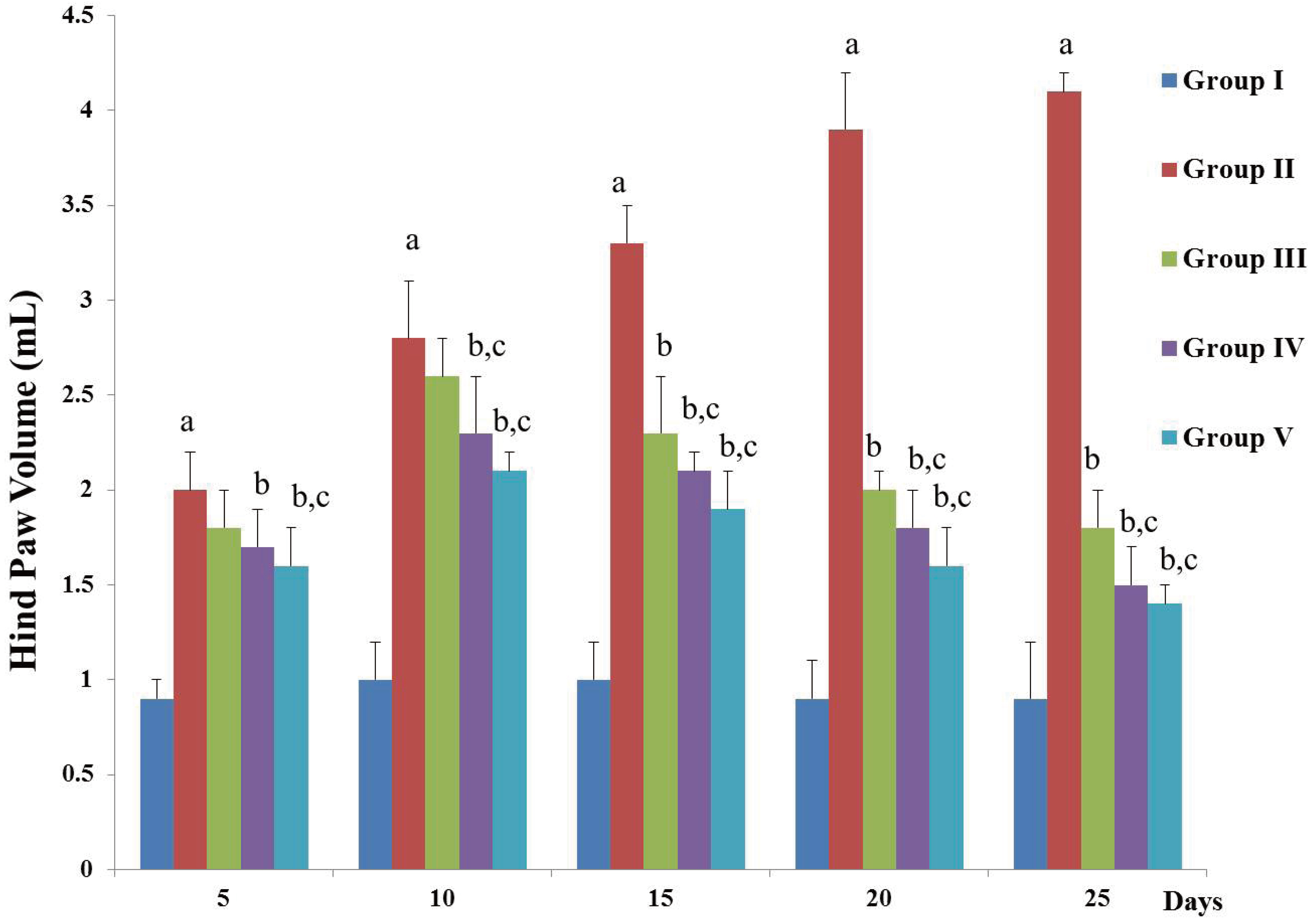
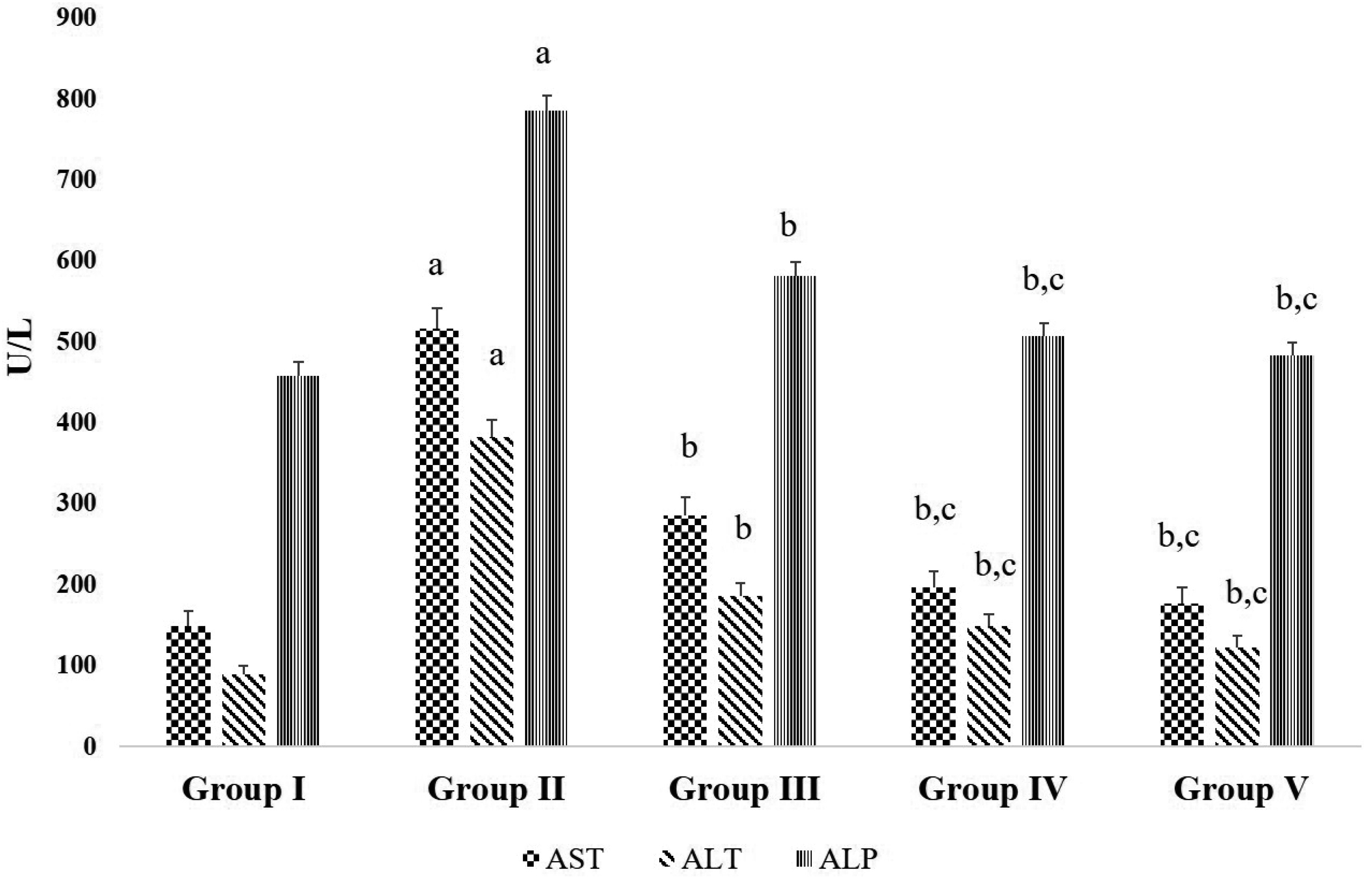
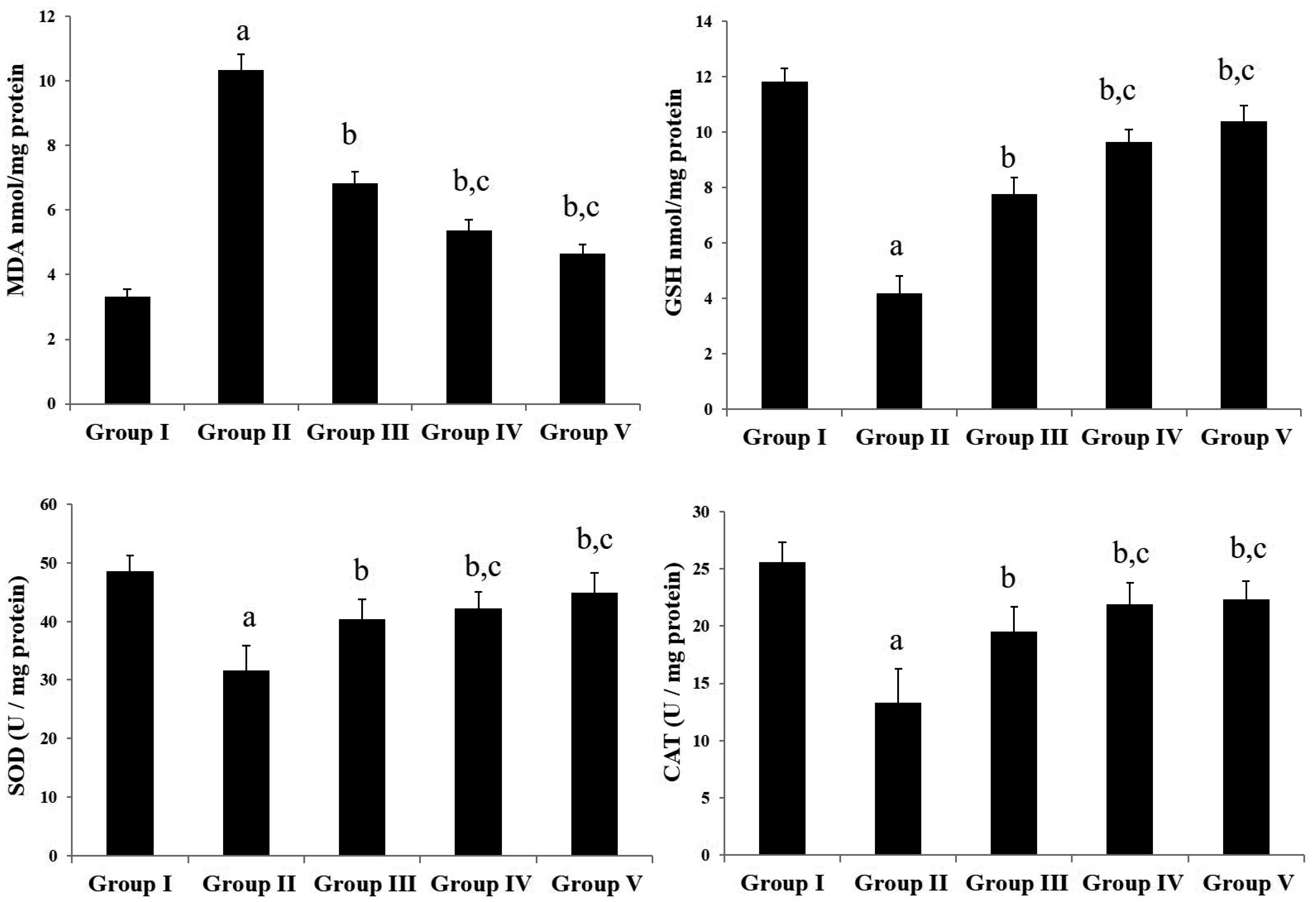
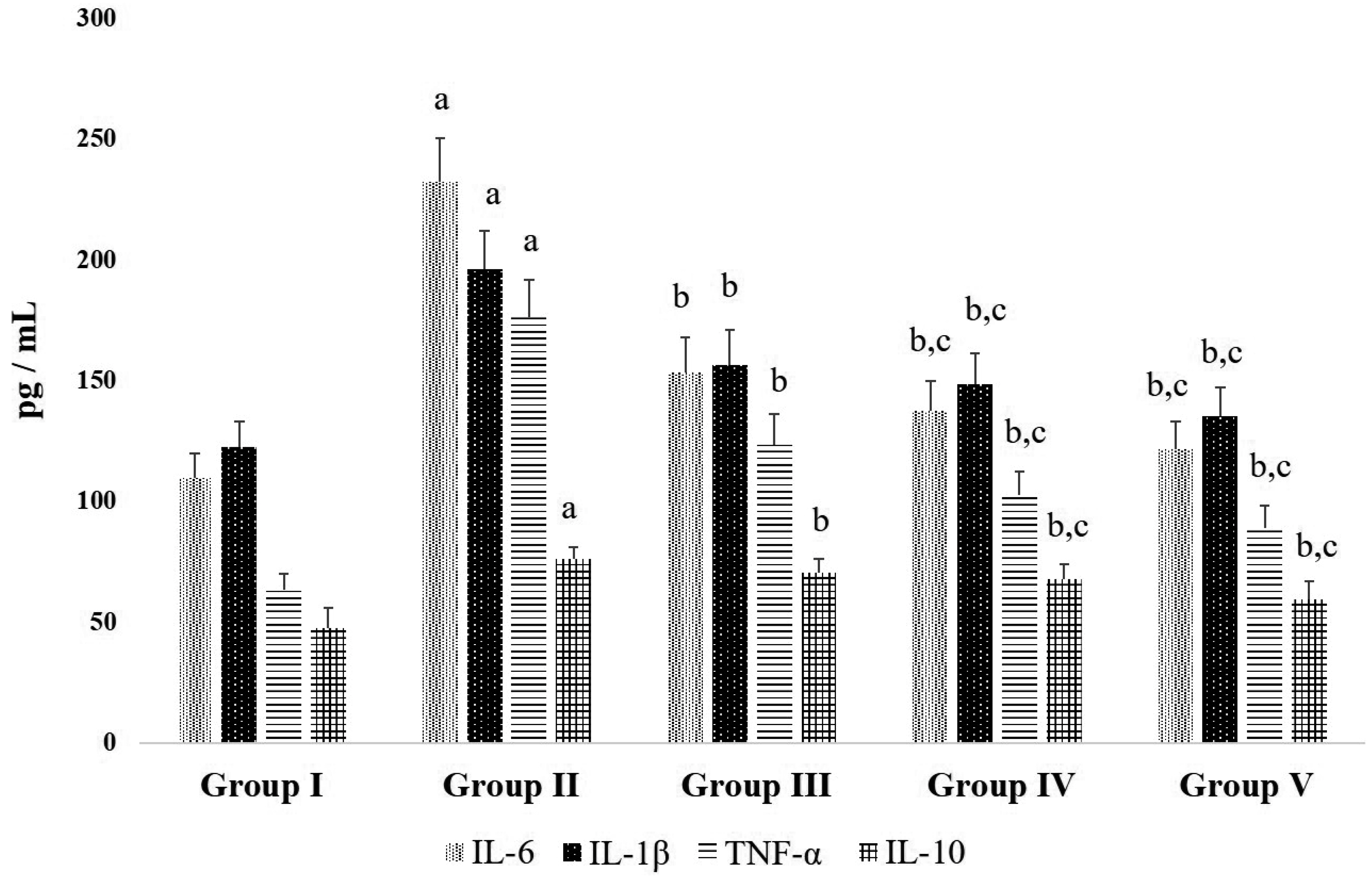
- Chronic joint pain due to loss of cartilage function, degradation of subchondral bone, and related conditions are common plights of an arthritis patient. Antioxidant compounds could solve the problems in arthritic condition. The objective of this study was to evaluate the anti-arthritic activity of D-carvone against complete Freund’s adjuvant (CFA)-induced arthritis in rats. D-carvone was orally administered for 25 days at the doses of 30 and 60 mg/kg against CFA-induced arthritic rats. Changes in body weight, paw swelling, organ index, hematological parameters, oxidative stress markers, inflammatory cytokines, and histopathology were recorded. Oral treatment of D-carvone significantly improved the body weight, reduced the paw swelling, edema formation, and organ index in arthritic rats. The levels of white blood cells were reduced, red blood cells and hemoglobin levels were improved in D-carvone treated arthritic rats. Lipid peroxidation levels were lowered whereas enzymatic and non-enzymatic antioxidants were significantly elevated by D-carvone administration against arthritic rats. D-carvone significantly modulated inflammatory cytokine levels and improved the ankle joint pathology against CFA-induced arthritic inflammation. In conclusion, D-carvone proved significant anti-arthritic activity against CFA-induced arthritis in rats.
Keyword
Figure
Reference
-
1. Poulet B, Staines KA. 2016; New developments in osteoarthritis and cartilage biology. Curr Opin Pharmacol. 28:8–13. DOI: 10.1016/j.coph.2016.02.009. PMID: 26921602.
Article2. Blasioli DJ, Kaplan DL. 2014; The roles of catabolic factors in the development of osteoarthritis. Tissue Eng Part B Rev. 20:355–363. DOI: 10.1089/ten.teb.2013.0377. PMID: 24172137. PMCID: PMC4123463.
Article3. Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. 2014; The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014:561459. DOI: 10.1155/2014/561459. PMID: 24876674. PMCID: PMC4021678.
Article4. Wang S, Wang Y, Liu X, Guan L, Yu L, Zhang X. 2016; Anti-inflammatory and anti-arthritic effects of taraxasterol on adjuvant-induced arthritis in rats. J Ethnopharmacol. 187:42–48. DOI: 10.1016/j.jep.2016.04.031. PMID: 27109342.
Article5. Jeong JH, Moon SJ, Jhun JY, Yang EJ, Cho ML, Min JK. 2015; Eupatilin exerts antinociceptive and chondroprotective properties in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. PLoS One. 10:e0130882. DOI: 10.1371/journal.pone.0130882. PMID: 26083352. PMCID: PMC4471346.
Article6. Park JS, Kim DK, Shin HD, Lee HJ, Jo HS, Jeong JH, Choi YL, Lee CJ, Hwang SC. 2016; Apigenin regulates interleukin-1β-induced production of matrix metalloproteinase both in the knee joint of rat and in primary cultured articular chondrocytes. Biomol Ther (Seoul). 24:163–170. DOI: 10.4062/biomolther.2015.217. PMID: 26902085. PMCID: PMC4774497.
Article7. Wang SX, Abramson SB, Attur M, Karsdal MA, Preston RA, Lozada CJ, Kosloski MP, Hong F, Jiang P, Saltarelli MJ, Hendrickson BA, Medema JK. 2017; Safety, tolerability, and pharmacodynamics of an anti-interleukin-1α/β dual variable domain immunoglobulin in patients with osteoarthritis of the knee: a randomized phase 1 study. Osteoarthritis Cartilage. 25:1952–1961. DOI: 10.1016/j.joca.2017.09.007. PMID: 28964890.
Article8. Wu Y, Goh EL, Wang D, Ma S. 2018; Novel treatments for osteoarthritis: an update. Open Access Rheumatol. 10:135–140. DOI: 10.2147/OARRR.S176666. PMID: 30323693. PMCID: PMC6174890.9. Huang X, Xi Y, Pan Q, Mao Z, Zhang R, Ma X, You H. 2018; Caffeic acid protects against IL-1β-induced inflammatory responses and cartilage degradation in articular chondrocytes. Biomed Pharmacother. 107:433–439. DOI: 10.1016/j.biopha.2018.07.161. PMID: 30103115.
Article10. Lin B, Zhao Y, Han P, Yue W, Ma XQ, Rahman K, Zheng CJ, Qin LP, Han T. 2014; Anti-arthritic activity of Xanthium strumarium L. extract on complete Freund̕s adjuvant induced arthritis in rats. J Ethnopharmacol. 155:248–255. DOI: 10.1016/j.jep.2014.05.023. PMID: 24862493.11. Gopalakrishnan T, Ganapathy S, Veeran V, Namasivayam N. 2019; Preventive effect of D-carvone during DMBA induced mouse skin tumorigenesis by modulating xenobiotic metabolism and induction of apoptotic events. Biomed Pharmacother. 111:178–187. DOI: 10.1016/j.biopha.2018.12.071. PMID: 30583225.
Article12. Vinothkumar R, Sudha M, Viswanathan P, Kabalimoorthy J, Balasubramanian T, Nalini N. 2013; Modulating effect of D-carvone on 1,2-dimethylhydrazine-induced pre-neoplastic lesions, oxidative stress and biotransforming enzymes, in an experimental model of rat colon carcinogenesis. Cell Prolif. 46:705–720. DOI: 10.1111/cpr.12062. PMID: 24118219.13. Moro IJ, Gondo GD, Pierri EG, Pietro RC, Soares CP, Sousa DP, dos Santos AG. 2018; Evaluation of antimicrobial, cytotoxic and chemopreventive activities of carvone and its derivatives. Braz J Pharm Sci. 53:e00076. DOI: 10.1590/s2175-97902017000400076.
Article14. Andrade LN, de Sousa DP. de Cássia da Silveira e Sá R. 2013; A review on anti-inflammatory activity of monoterpenes. Molecules. 18:1227–1254. DOI: 10.3390/molecules18011227. PMID: 23334570. PMCID: PMC6269770.
Article15. Chen Y, Xue R, Jin X, Tan X. 2018; Antiarthritic activity of diallyl disulfide against Freund's adjuvant-induced arthritic rat model. J Environ Pathol Toxicol Oncol. 37:291–303. DOI: 10.1615/JEnvironPatholToxicolOncol.2018027078. PMID: 30806236.
Article16. Rahmati M, Mobasheri A, Mozafari M. 2016; Inflammatory mediators in osteoarthritis: a critical review of the state-of-the-art, current prospects, and future challenges. Bone. 85:81–90. DOI: 10.1016/j.bone.2016.01.019. PMID: 26812612.
Article17. Patil MVK, Kandhare AD, Bhise SD. 2012; Anti-arthritic and anti-inflammatory activity of Xanthium srtumarium L. ethanolic extract in Freund's complete adjuvant induced arthritis. Biomed Aging Pathol. 2:6–15. DOI: 10.1016/j.biomag.2012.01.002.18. Cui X, Wang R, Bian P, Wu Q, Seshadri VDD, Liu L. 2019; Evaluation of antiarthritic activity of nimbolide against Freund's adjuvant induced arthritis in rats. Artif Cells Nanomed Biotechnol. 47:3391–3398. DOI: 10.1080/21691401.2019.1649269. PMID: 31394949.
Article19. Zhang X, Dong Y, Dong H, Zhang W, Li F. 2017; Investigation of the effect of phlomisoside F on complete Freund's adjuvant-induced arthritis. Exp Ther Med. 13:710–716. DOI: 10.3892/etm.2016.3995. PMID: 28352356. PMCID: PMC5348710.
Article20. Lima-Garcia JF, Dutra RC, da Silva K, Motta EM, Campos MM, Calixto JB. 2011; The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol. 164:278–293. DOI: 10.1111/j.1476-5381.2011.01345.x. PMID: 21418187. PMCID: PMC3174409.
Article21. Zhang L, Zhu M, Li M, Du Y, Duan S, Huang Y, Lu Y, Zhang J, Wang T, Fu F. 2017; Ginsenoside Rg1 attenuates adjuvant-induced arthritis in rats via modulation of PPAR-γ/NF-κB signal pathway. Oncotarget. 8:55384–55393. DOI: 10.18632/oncotarget.19526. PMID: 28903427. PMCID: PMC5589666.
Article22. Qiao YQ, Jiang PF, Gao YZ. 2018; Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Arch Med Sci. 14:617–624. DOI: 10.5114/aoms.2016.59871. PMID: 29765450. PMCID: PMC5949909.
Article23. Hou SM, Hou CH, Liu JF. 2017; CX3CL1 promotes MMP-3 production via the CX3CR1, c-Raf, MEK, ERK, and NF-κB signaling pathway in osteoarthritis synovial fibroblasts. Arthritis Res Ther. 19:282. DOI: 10.1186/s13075-017-1487-6. PMID: 29268768. PMCID: PMC5740560.
Article24. Jiang L, Li L, Geng C, Gong D, Jiang L, Ishikawa N, Kajima K, Zhong L. 2013; Monosodium iodoacetate induces apoptosis via the mitochondrial pathway involving ROS production and caspase activation in rat chondrocytes in vitro. J Orthop Res. 31:364–369. DOI: 10.1002/jor.22250. PMID: 23124986.25. Zhao M, Du J. 2020; Anti-inflammatory and protective effects of D-carvone on lipopolysaccharide (LPS)-induced acute lung injury in mice. J King Saud Univ Sci. 32:1592–1596. DOI: 10.1016/j.jksus.2019.12.016.
Article26. Marchese A, Arciola CR, Barbieri R, Silva AS, Nabavi SF, Tsetegho Sokeng AJ, Izadi M, Jafari NJ, Suntar I, Daglia M, Nabavi SM. 2017; Update on monoterpenes as antimicrobial agents: a particular focus on p-cymene. Materials (Basel). 10:947. DOI: 10.3390/ma10080947. PMID: 28809799. PMCID: PMC5578313.
Article27. Pavan B, Dalpiaz A, Marani L, Beggiato S, Ferraro L, Canistro D, Paolini M, Vivarelli F, Valerii MC, Comparone A, De Fazio L, Spisni E. 2018; Geraniol pharmacokinetics, bioavailability and its multiple effects on the liver antioxidant and xenobiotic-metabolizing enzymes. Front Pharmacol. 9:18. DOI: 10.3389/fphar.2018.00018. PMID: 29422862. PMCID: PMC5788896.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory, Anti-arthritic and Analgesic Effect of the Herbal Extract Made from Bacopa monnieriis, Cassia fistula and Phyllanthus polyphyllus
- Antinociceptive Effects of Prim-O-Glucosylcimifugin in Inflammatory Nociception via Reducing Spinal COX-2
- Vitamin E Reduces Symptoms in a CFA-induced Model of Monoarthritis
- Prophylactic effect of plaster and cataplasm contained ketoprofen in rats with adjuvant arthritis
- Anti-arthritic Effects of Oplopanax elatus in a Rat Model of Rheumatoid Arthritis (Adjuvant-induced Arthritis)