Nat Prod Sci.
2019 Dec;25(4):304-310. 10.20307/nps.2019.25.4.304.
Anti-arthritic Effects of Oplopanax elatus in a Rat Model of Rheumatoid Arthritis (Adjuvant-induced Arthritis)
- Affiliations
-
- 1College of Pharmacy, Kangwon National University, Chuncheon 24341, Korea. hpkim@kangwon.ac.kr
- 2Department of Bio-Resource Sciences, Kangwon National University, Chuncheon 24341, Korea.
- 3Bioherb Research Institute, Kangwon National University, Chuncheon 24341, Korea.
- KMID: 2468063
- DOI: http://doi.org/10.20307/nps.2019.25.4.304
Abstract
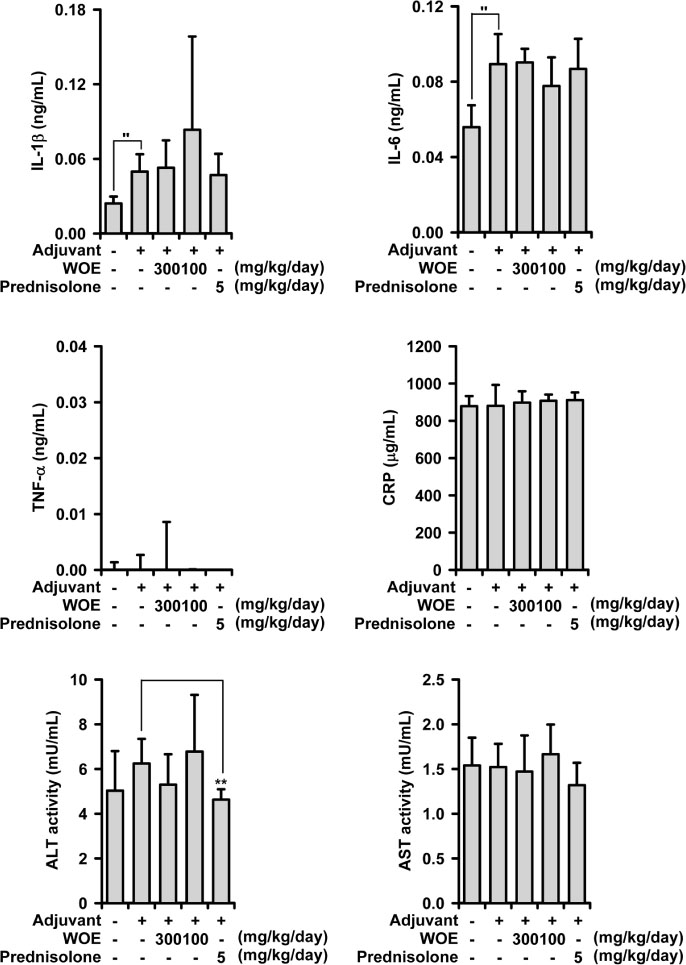
- The stems of Oplopanax elatus (OE) have long been used to treat inflammatory disorders in herbal medicine, and in the previous investigation, OE was found to possess anti-inflammatory activity in lipopolysaccharide-treated macrophages, RAW 264.7 cell. OE reduces inducible nitric oxide (NO) synthase-induced NO production, and interferes with mitogen-activated protein kinase activation pathways. In the present study, the pharmacological action of the water extract of OE was examined to establish anti-arthritic action, using a rat model of adjuvant-induced arthritis (AIA). The water extract of OE administered orally inhibited AIA-induced arthritis at (100 - 300) mg/kg/day. The paw edema was significantly decreased, in combination with reduced production of pro-inflammatory cytokines. The action mechanism includes an inhibition of MAPKs/nuclear transcription factor-κB activation. These new findings strongly suggest that OE possesses anti-arthritic action, and may be used as a therapeutic agent in inflammation-related disorders, particularly in arthritic condition.
Keyword
MeSH Terms
Figure
Reference
-
1. Crofford LJ. Arthritis Res Ther. 2013; 15:S2.
Article2. Ong CK, Lirk P, Tan CH, Seymou RA. Clin Med Res. 2007; 5:19–34.3. Kang S, Tanaka T, Kishimoto T. Int Immunol. 2015; 27:21–29.4. Kumar S, Bhosle D, Janghel A, Deo S, Raut P, Verma C, Agrawal M, Amit N, Sharma M, Giri T, Tripathi DK, Ajaz A, Alexander A. Res J Pharm Tech. 2015; 8:597–610.5. Shikov AN, Pozharitskaya ON, Makarov VG, Yang WZ, Guo DA. Chin J Nat Med. 2014; 12:721–729.6. Zhong Hua Ben Cao Commission. Chinese Materia Medica (Zhong Hua Ben Cao). China: Shanghai Science and Technology Press;1999. p. 5026–5027.7. Zhang SC, Wang KZ. Acta Pharm Sin. 1980; 15:81–85.8. Qu SY, Jiang XL, Wu YJ, Wang YH. Chin Tradit Herb Drugs. 1986; 17:25–26.9. Kim HK, Son KH, Chang HW, Kang SS, Kim HP. Planta Med. 1999; 65:465–467.10. Choi J, Shin KM, Park HJ, Jung HJ, Kim HJ, Lee YS, Rew JH, Lee KT. Planta Med. 2004; 70:1027–1032.11. Zhang A, Liu Z, Sheng L, Wu H. J Surg Res. 2017; 209:252–257.12. Kwon KS, Lee JH, So KS, Park BK, Lim H, Choi JS, Kim HP. Phytother Res. 2018; 32:1537–1545.13. Zhu T, Zhang W, Feng SJ, Yu HP. Int Immunopharmacol. 2016; 34:16–24.14. Yang MC, Kwon HC, Kim YJ, Lee KR, Yang HO. J Nat Prod. 2010; 73:801–805.15. Kim SY, Son KH, Chang HW, Kang SS, Kim HP. Arch Pharm Res. 1997; 20:313–317.16. Bihani GV, Rojatkar SR, Bodhankar SL. Biomed Aging Pathol. 2014; 4:197–206.17. Srivastava S, Singh P, Jha KK, Mishra G, Srivastava S, Khosa RL. J Ayurveda Integr Med. 2012; 3:204–208.