Anat Cell Biol.
2020 Sep;53(3):355-365. 10.5115/acb.18.169.
Curcumin supplementation shows modulatory influence on functional and morphological features of hippocampus in mice subjected to arsenic trioxide exposure
- Affiliations
-
- 1Department of Anatomy, All India Institute of Medical Sciences, New Delhi, India
- KMID: 2507648
- DOI: http://doi.org/10.5115/acb.18.169
Abstract
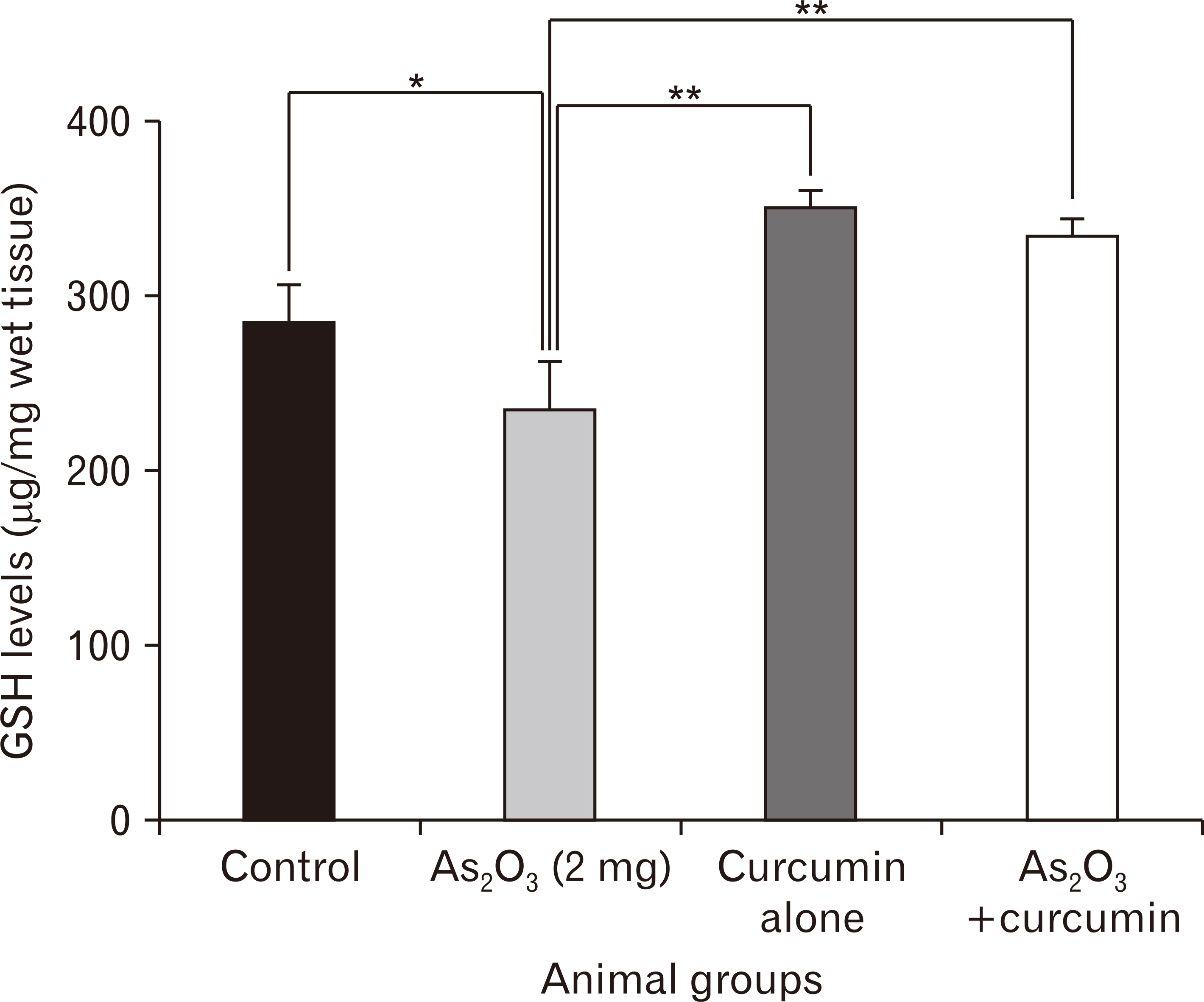
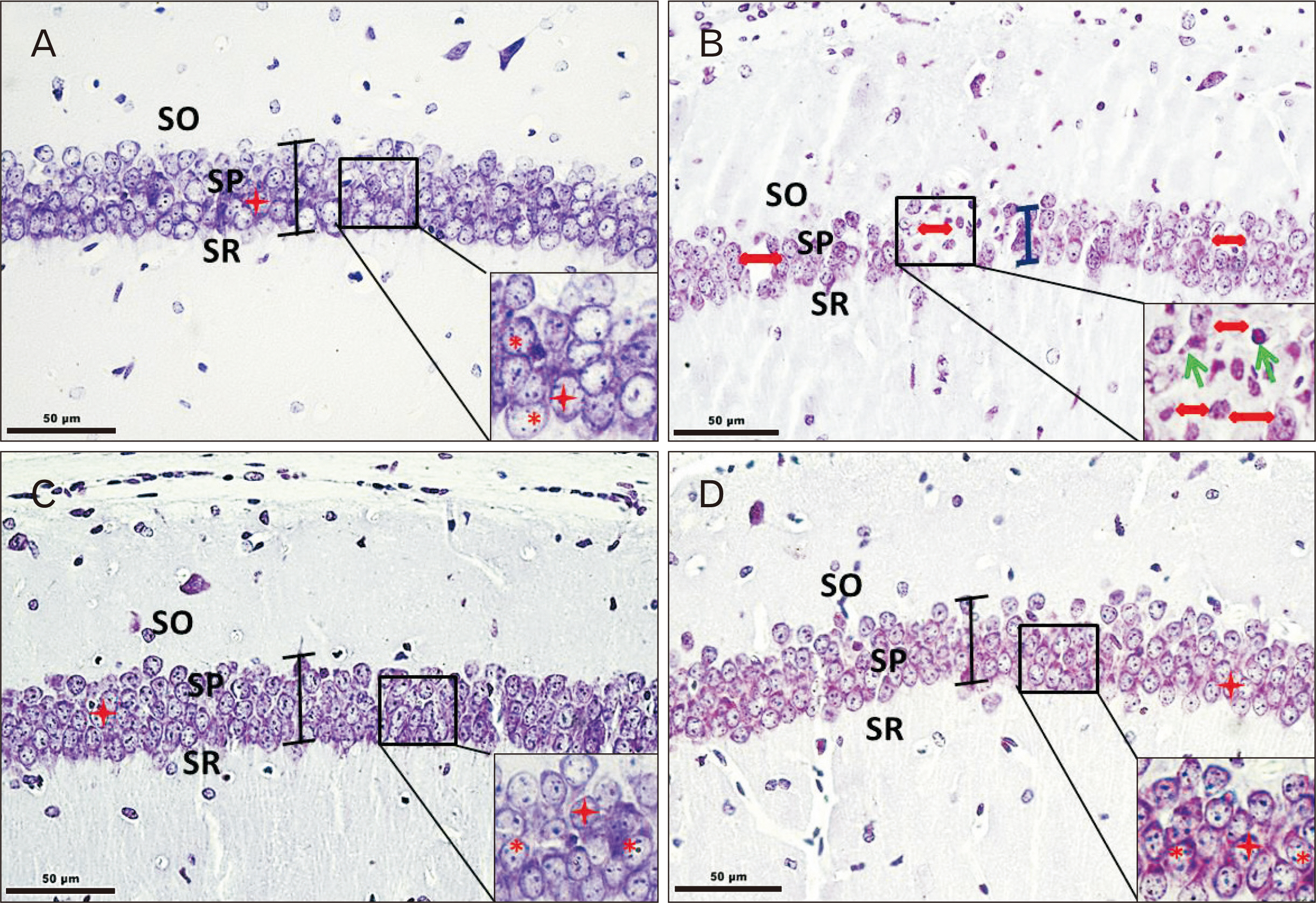
- Since, oxidative stress has been suggested as one of the mechanisms underlying arsenic-induced toxicity, the present study focused on the role of antioxidant (curcumin) supplementation on behavioral, biochemical, and morphological alterations with context to mice hippocampus (CA1) following arsenic trioxide (As2O3) administration. Healthy male Swiss albino mice were divided into control and experimental groups. As2O3 (2 mg/kg bw) alone or along with curcumin (100 mg/kg bw) was administered to experimental groups by oral route for 45 days whereas the control groups received either no treatment or vehicle for curcumin. Animals were subjected to behavioral study towards the end of the experimental period (day 33–45). On day 46, the brain samples were obtained and subjected either to immersion fixation (for morphometric observations) or used afresh for biochemical test. Behavioral tests (open field, elevated plus maze, and Morris water maze) revealed enhanced anxiety levels and impairment of cognitive functions in As2O3 alone treated groups whereas a trend of recovery was evident in mice simultaneously treated with As2O3 and curcumin. Morphological observations showed noticeable reduction in stratum pyramidale thickness (CA1), along with decrease in density and size of pyramidal neurons in As2O3 alone exposed group as compared to As2O3 +Cu co-treated group. Hippocampal glutathione levels were found to be downregulated in animals receiving As2O3 as against the levels of controls and curcumin supplemented animals, thereby, suggestive of beneficial role of curcumin on As2O3 induced adverse effects.
Figure
Reference
-
References
1. Vahidnia A, van der Voet GB, de Wolff FA. 2007; Arsenic neurotoxicity: a review. Hum Exp Toxicol. 26:823–32. DOI: 10.1177/0960327107084539. PMID: 18025055.2. Agency for Toxic Substances and Disease Registry. 2007. Toxicological profile for arsenic. Public Health Service, Agency for Toxic Substances and Disease Registry;Atlanta, GA:3. Brinkel J, Khan MH, Kraemer A. 2009; A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh. Int J Environ Res Public Health. 6:1609–19. DOI: 10.3390/ijerph6051609. PMID: 19543409. PMCID: PMC2697931.
Article4. Rao Y, Li R, Zhang D. 2013; A drug from poison: how the therapeutic effect of arsenic trioxide on acute promyelocytic leukemia was discovered. Sci China Life Sci. 56:495–502. DOI: 10.1007/s11427-013-4487-z. PMID: 23645104.
Article5. Iland HJ, Seymour JF. 2013; Role of arsenic trioxide in acute promyelocytic leukemia. Curr Treat Options Oncol. 14:170–84. DOI: 10.1007/s11864-012-0223-3. PMID: 23322117.
Article6. Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, Kim BK, Lee YY. 2000; Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res. 60:3065–71. PMID: 10850458.7. Rojewski MT, Baldus C, Knauf W, Thiel E, Schrezenmeier H. 2002; Dual effects of arsenic trioxide (As2O3) on non-acute promyelocytic leukaemia myeloid cell lines: induction of apoptosis and inhibition of proliferation. Br J Haematol. 116:555–63. DOI: 10.1046/j.0007-1048.2001.03298.x. PMID: 11849211.
Article8. Hussein MA, Saleh M, Ravandi F, Mason J, Rifkin RM, Ellison R. 2004; Phase 2 study of arsenic trioxide in patients with relapsed or refractory multiple myeloma. Br J Haematol. 125:470–6. DOI: 10.1111/j.1365-2141.2004.04941.x. PMID: 15142117.
Article9. Wang L, Wang R, Fan L, Liang W, Liang K, Xu Y, Peng G, Ye Q. 2017; Arsenic trioxide is an immune adjuvant in liver cancer treatment. Mol Immunol. 81:118–26. DOI: 10.1016/j.molimm.2016.12.001. PMID: 27940255.
Article10. Novick SC. 2000; Arsenicals in hematologic cancers. Semin Oncol. 27:495–501.11. Lengfelder E, Hofmann WK, Nowak D. 2012; Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia. 26:433–42. DOI: 10.1038/leu.2011.245. PMID: 21904379.
Article12. Emadi A, Gore SD. 2010; Arsenic trioxide: an old drug rediscovered. Blood Rev. 24:191–9. DOI: 10.1016/j.blre.2010.04.001. PMID: 20471733.13. Kritharis A, Bradley TP, Budman DR. 2013; The evolving use of arsenic in pharmacotherapy of malignant disease. Ann Hematol. 92:719–30. DOI: 10.1007/s00277-013-1707-3. PMID: 23494203.
Article14. Fanselow MS, Dong HW. 2010; Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 65:7–19. DOI: 10.1016/j.neuron.2009.11.031. PMID: 20152109. PMCID: PMC2822727.
Article15. Kosaki Y, Lin TC, Horne MR, Pearce JM, Gilroy KE. 2014; The role of the hippocampus in passive and active spatial learning. Hippocampus. 24:1633–52. DOI: 10.1002/hipo.22343. PMID: 25131441. PMCID: PMC4258078.
Article16. Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE, Weisman GA, Sun GY. 2005; Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 82:138–48. DOI: 10.1002/jnr.20610. PMID: 16075466. PMCID: PMC5822585.
Article17. Wang X, Michaelis EK. 2010; Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2:12. DOI: 10.3389/fnagi.2010.00012. PMID: 20552050.
Article18. Griffin AL. 2015; Role of the thalamic nucleus reuniens in mediating interactions between the hippocampus and medial prefrontal cortex during spatial working memory. Front Syst Neurosci. 9:29. DOI: 10.3389/fnsys.2015.00029. PMID: 25805977. PMCID: PMC4354269.
Article19. Zhang NY, Qi M, Zhao L, Zhu MK, Guo J, Liu J, Gu CQ, Rajput SA, Krumm CS, Qi DS, Sun LH. 2016; Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver. Toxins (Basel). 8:E327. DOI: 10.3390/toxins8110327. PMID: 27834912.
Article20. Da Silva Morrone M, Schnorr CE, Behr GA, Gasparotto J, Bortolin RC, Moresco KS, Bittencourt L, Zanotto-Filho A, Gelain DP, Moreira JC. 2016; Oral administration of curcumin relieves behavioral alterations and oxidative stress in the frontal cortex, hippocampus, and striatum of ovariectomized Wistar rats. J Nutr Biochem. 32:181–8. DOI: 10.1016/j.jnutbio.2016.03.010. PMID: 27142750.
Article21. Sankar P, Telang AG, Kalaivanan R, Karunakaran V, Suresh S, Kesavan M. 2016; Oral nanoparticulate curcumin combating arsenic-induced oxidative damage in kidney and brain of rats. Toxicol Ind Health. 32:410–21. DOI: 10.1177/0748233713498455. PMID: 24105067.
Article22. Rajasekar N, Dwivedi S, Tota SK, Kamat PK, Hanif K, Nath C, Shukla R. 2013; Neuroprotective effect of curcumin on okadaic acid induced memory impairment in mice. Eur J Pharmacol. 715:381–94. DOI: 10.1016/j.ejphar.2013.04.033. PMID: 23685326.
Article23. Srivastava P, Yadav RS, Chandravanshi LP, Shukla RK, Dhuriya YK, Chauhan LK, Dwivedi HN, Pant AB, Khanna VK. 2014; Unraveling the mechanism of neuroprotection of curcumin in arsenic induced cholinergic dysfunctions in rats. Toxicol Appl Pharmacol. 279:428–40. DOI: 10.1016/j.taap.2014.06.006. PMID: 24952339.
Article24. Tatem KS, Quinn JL, Phadke A, Yu Q, Gordish-Dressman H, Nagaraju K. 2014; Gordish-Dressman H, Nagaraju K. Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. J Vis Exp. (91):51785. DOI: 10.3791/51785. PMID: 25286313. PMCID: PMC4672952.
Article25. Walf AA, Frye CA. 2007; The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2:322–8. DOI: 10.1038/nprot.2007.44. PMID: 17406592.
Article26. Morris R. 1984; Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 11:47–60. DOI: 10.1016/0165-0270(84)90007-4. PMID: 6471907. PMCID: PMC2566857.
Article27. Vorhees CV, Williams MT. 2006; Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 1:848–58. DOI: 10.1038/nprot.2006.116. PMID: 17406317. PMCID: PMC2895266.
Article28. Ellman GL. 1959; Tissue sulfhydryl groups. Arch Biochem Biophys. 82:70–7. DOI: 10.1016/0003-9861(59)90090-6.
Article29. Paxinos G, Franklin KB. 2008. The mouse brain in stereotaxic coordinates. Academic Press;San Diego, CA:30. Rhodes MC, Seidler FJ, Abdel-Rahman A, Tate CA, Nyska A, Rincavage HL, Slotkin TA. 2004; Terbutaline is a developmental neurotoxicant: effects on neuroproteins and morphology in cerebellum, hippocampus, and somatosensory cortex. J Pharmacol Exp Ther. 308:529–37. DOI: 10.1124/jpet.103.060095. PMID: 14610225.
Article31. Miki T, Harris SJ, Wilce PA, Takeuchi Y, Bedi KS. 2004; Effects of age and alcohol exposure during early life on pyramidal cell numbers in the CA1-CA3 region of the rat hippocampus. Hippocampus. 14:124–34. DOI: 10.1002/hipo.10155. PMID: 15058490.
Article32. Ferris NJ, Cragg BG. 1984; Organic lead and histological parameters of brain development. Acta Neuropathol. 63:306–12. DOI: 10.1007/BF00687338. PMID: 6475490. PMCID: PMC6590474.
Article33. Gundersen HJ. 1977; Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J Microsc. 111:219–23. DOI: 10.1111/j.1365-2818.1977.tb00062.x.
Article34. Isgor C, Kabbaj M, Akil H, Watson SJ. 2004; Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 14:636–48. DOI: 10.1002/hipo.10207. PMID: 15301440.
Article35. Anacker C, Hen R. 2017; Adult hippocampal neurogenesis and cognitive flexibility: linking memory and mood. Nat Rev Neurosci. 18:335–46. DOI: 10.1038/nrn.2017.45. PMID: 28469276.36. Evens AM, Tallman MS, Gartenhaus RB. 2004; The potential of arsenic trioxide in the treatment of malignant disease: past, present, and future. Leuk Res. 28:891–900. DOI: 10.1016/j.leukres.2004.01.011. PMID: 15234563.
Article37. Rust DM, Soignet SL. 2001; Risk/benefit profile of arsenic trioxide. Oncologist. 6 Suppl 2:29–32. DOI: 10.1634/theoncologist.6-suppl_2-29. PMID: 11331438.
Article38. Nachman KE, Baron PA, Raber G, Francesconi KA, Navas-Acien A, Love DC. 2013; Roxarsone, inorganic arsenic, and other arsenic species in chicken: a U.S.-based market basket sample. Environ Health Perspect. 121:818–24. DOI: 10.1289/ehp.1206245. PMID: 23694900. PMCID: PMC3701911.
Article39. Silbergeld EK, Nachman K. 2008; The environmental and public health risks associated with arsenical use in animal feeds. Ann N Y Acad Sci. 1140:346–57. DOI: 10.1196/annals.1454.049. PMID: 18991934. PMCID: PMC6941779.
Article40. Chang CY, Guo HR, Tsai WC, Yang KL, Lin LC, Cheng TJ, Chuu JJ. 2015; Subchronic arsenic exposure induces anxiety-like behaviors in normal mice and enhances depression-like behaviors in the chemically induced mouse model of depression. Biomed Res Int. 2015:159015. DOI: 10.1155/2015/159015. PMID: 26114099. PMCID: PMC4465655.
Article41. Bardullas U, Limón-Pacheco JH, Giordano M, Carrizales L, Mendoza-Trejo MS, Rodríguez VM. 2009; Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol Appl Pharmacol. 239:169–77. DOI: 10.1016/j.taap.2008.12.004. PMID: 19121333.
Article42. Rodríguez VM, Jiménez-Capdeville ME, Giordano M. 2003; The effects of arsenic exposure on the nervous system. Toxicol Lett. 145:1–18. DOI: 10.1016/S0378-4274(03)00262-5.
Article43. López Ruiz JR, Osuna Carrasco LP, López Valenzuela CL, Franco Rodríguez NE, de la Torre Valdovinos B, Jiménez Estrada I, Dueñas Jiménez JM, Dueñas Jiménez SH. 2015; The hippocampus participates in the control of locomotion speed. Neuroscience. 311:207–15. DOI: 10.1016/j.neuroscience.2015.10.034. PMID: 26597762. PMCID: PMC5376603.
Article44. Lu CB, Henderson Z. 2010; Nicotine induction of theta frequency oscillations in rodent hippocampus in vitro. Neuroscience. 166:84–93. DOI: 10.1016/j.neuroscience.2009.11.072. PMID: 20004706.45. Fuhrmann F, Justus D, Sosulina L, Kaneko H, Beutel T, Friedrichs D, Schoch S, Schwarz MK, Fuhrmann M, Remy S. 2015; Locomotion, theta oscillations, and the speed-correlated firing of hippocampal neurons are controlled by a medial septal glutamatergic circuit. Neuron. 86:1253–64. DOI: 10.1016/j.neuron.2015.05.001. PMID: 25982367.
Article46. Cenci MA. 2007; Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 30:236–43. DOI: 10.1016/j.tins.2007.03.005. PMID: 17400300.
Article47. Naqvi F, Haider S, Batool Z, Perveen T, Haleem DJ. 2012; Sub-chronic exposure to noise affects locomotor activity and produces anxiogenic and depressive like behavior in rats. Pharmacol Rep. 64:64–9. DOI: 10.1016/S1734-1140(12)70731-4. PMID: 22580521.
Article48. Rosado JL, Ronquillo D, Kordas K, Rojas O, Alatorre J, Lopez P, Garcia-Vargas G, Del Carmen Caamaño M, Cebrián ME, Stoltzfus RJ. 2007; Arsenic exposure and cognitive performance in Mexican schoolchildren. Environ Health Perspect. 115:1371–5. DOI: 10.1289/ehp.9961. PMID: 17805430. PMCID: PMC1964916.
Article49. Tyler CR, Allan AM. 2014; The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep. 1:132–47. DOI: 10.1007/s40572-014-0012-1. PMID: 24860722. PMCID: PMC4026128.
Article50. Adedayo AD, Aderinola AA, Adekilekun TA, Olaolu OO, Olanike AM, Olayemi IK. 2018; Morphine-alcohol treatment impairs cognitive functions and increases neuro-inflammatory responses in the medial prefrontal cortex of juvenile male rats. Anat Cell Biol. 51:41–51. DOI: 10.5115/acb.2018.51.1.41. PMID: 29644109. PMCID: PMC5890016.
Article51. Jiang S, Su J, Yao S, Zhang Y, Cao F, Wang F, Wang H, Li J, Xi S. 2014; Fluoride and arsenic exposure impairs learning and memory and decreases mGluR5 expression in the hippocampus and cortex in rats. PLoS One. 9:e96041. DOI: 10.1371/journal.pone.0096041. PMID: 24759735. PMCID: PMC3997496.
Article52. Ramos-Chávez LA, Rendón-López CR, Zepeda A, Silva-Adaya D, Del Razo LM, Gonsebatt ME. 2015; Neurological effects of inorganic arsenic exposure: altered cysteine/glutamate transport, NMDA expression and spatial memory impairment. Front Cell Neurosci. 9:21. DOI: 10.3389/fncel.2015.00021. PMID: 25709567. PMCID: PMC4321597.
Article53. Lucetti EC, Lucetti DL, da Silva Ribeiro AE, de Moura RB, Sampaio TM, de Almeida VL, Sérvula , da Silva AS, Bezerra LR, Neves KR, de Barros Viana GS. 2016; Curcumin reversion of neurochemical and immunohistochemical alterations in brain ischemia is related to its antioxidant and anti-inflammatory properties. J Med Plants Stud. 4:20–9.54. Kitchin KT, Conolly R. 2010; Arsenic-induced carcinogenesis--oxidative stress as a possible mode of action and future research needs for more biologically based risk assessment. Chem Res Toxicol. 23:327–35. DOI: 10.1021/tx900343d. PMID: 20035570.
Article55. Tolins M, Ruchirawat M, Landrigan P. 2014; The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann Glob Health. 80:303–14. DOI: 10.1016/j.aogh.2014.09.005. PMID: 25459332.
Article56. Shi H, Shi X, Liu KJ. 2004; Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem. 255:67–78. DOI: 10.1023/B:MCBI.0000007262.26044.e8. PMID: 14971647.
Article57. Yen YP, Tsai KS, Chen YW, Huang CF, Yang RS, Liu SH. 2012; Arsenic induces apoptosis in myoblasts through a reactive oxygen species-induced endoplasmic reticulum stress and mitochondrial dysfunction pathway. Arch Toxicol. 86:923–33. DOI: 10.1007/s00204-012-0864-9. PMID: 22622864.
Article58. Flora SJ, Mittal M, Mehta A. 2008; Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res. 128:501–23.59. Barzegar A, Moosavi-Movahedi AA. 2011; Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS One. 6:e26012. DOI: 10.1371/journal.pone.0026012. PMID: 22016801. PMCID: PMC3189944.
Article60. Bartsch T, Alfke K, Stingele R, Rohr A, Freitag-Wolf S, Jansen O, Deuschl G. 2006; Selective affection of hippocampal CA-1 neurons in patients with transient global amnesia without long-term sequelae. Brain. 129(Pt 11):2874–84. DOI: 10.1093/brain/awl248. PMID: 17003071.
Article61. Wang JY, Xia Q, Chu KT, Pan J, Sun LN, Zeng B, Zhu YJ, Wang Q, Wang K, Luo BY. 2011; Severe global cerebral ischemia-induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3-methyladenine: a widely used inhibitor of autophagy. J Neuropathol Exp Neurol. 70:314–22. DOI: 10.1097/NEN.0b013e31821352bd. PMID: 21412169.
Article62. Wang X, Zaidi A, Pal R, Garrett AS, Braceras R, Chen XW, Michaelis ML, Michaelis EK. 2009; Genomic and biochemical approaches in the discovery of mechanisms for selective neuronal vulnerability to oxidative stress. BMC Neurosci. 10:12. DOI: 10.1186/1471-2202-10-12. PMID: 19228403. PMCID: PMC2677396.
Article63. Schipper HM, Lee JS, Singer J, Waxman S. 2002; Mechanisms of action of arsenic trioxide. Cancer Res. 62:3893–903. DOI: 10.5353/th_b3689998.64. Wang Y, Bai C, Guan H, Chen R, Wang X, Wang B, Jin H, Piao F. 2015; Subchronic exposure to arsenic induces apoptosis in the hippocampus of the mouse brains through the Bcl-2/Bax pathway. J Occup Health. 57:212–21. DOI: 10.1539/joh.14-0226-OA. PMID: 25787108.
Article65. Luo J, Qiu Z, Chen J, Zhang L, Liu W, Tan Y, Shu W. 2013; Maternal and early life arsenite exposure impairs neurodevelopment and increases the expression of PSA-NCAM in hippocampus of rat offspring. Toxicology. 311:99–106. DOI: 10.1016/j.tox.2013.06.007. PMID: 23811142.
Article66. Dhar P, Mohari N, Mehra RD. 2007; Preliminary morphological and morphometric study of rat cerebellum following sodium arsenite exposure during rapid brain growth (RBG) period. Toxicology. 234:10–20. DOI: 10.1016/j.tox.2007.01.024. PMID: 17374429.
Article67. Frankel S, Concannon J, Brusky K, Pietrowicz E, Giorgianni S, Thompson WD, Currie DA. 2009; Arsenic exposure disrupts neurite growth and complexity in vitro. Neurotoxicology. 30:529–37. DOI: 10.1016/j.neuro.2009.02.015. PMID: 19635389.68. Wang X, Meng D, Chang Q, Pan J, Zhang Z, Chen G, Ke Z, Luo J, Shi X. 2010; Arsenic inhibits neurite outgrowth by inhibiting the LKB1-AMPK signaling pathway. Environ Health Perspect. 118:627–34. DOI: 10.1289/ehp.0901510. PMID: 20439172. PMCID: PMC2866677.
Article69. Jia T, Sun Z, Lu Y, Gao J, Zou H, Xie F, Zhang G, Xu H, Sun D, Yu Y, Zhong Y. 2016; A dual brain-targeting curcumin-loaded polymersomes ameliorated cognitive dysfunction in intrahippocampal amyloid-β1-42-injected mice. Int J Nanomedicine. 11:3765–75. DOI: 10.2147/IJN.S94622. PMID: 27540290. PMCID: PMC4981163.70. Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, Chung HY, Mattson MP, Lee J. 2008; Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 283:14497–505. DOI: 10.1074/jbc.M708373200. PMID: 18362141. PMCID: PMC2386914.
Article71. Wang X, Pal R, Chen XW, Limpeanchob N, Kumar KN, Michaelis EK. 2005; High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res Mol Brain Res. 140:120–6. DOI: 10.1016/j.molbrainres.2005.07.018. PMID: 16137784.
Article72. Wu J, Li Q, Wang X, Yu S, Li L, Wu X, Chen Y, Zhao J, Zhao Y. 2013; Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS One. 8:e59843. DOI: 10.1371/journal.pone.0059843. PMID: 23555802. PMCID: PMC3610879.
Article73. Cui Q, Li X, Zhu H. 2016; Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Mol Med Rep. 13:1381–8. DOI: 10.3892/mmr.2015.4657. PMID: 26648392.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Osteomyelitis on Maxilla Caused by Arsenic Trioxide
- Antitumor Effects of Arsenic Trioxide on Neuroblastoma
- A Case of Relapsed Acute Promyleocytic Leukemia Induced Remission with Arsenic Trioxide(As2O3)
- Arsenic Trioxide, an Old Drug? or a New Drug?
- Anticancer Effect of Arsenic Trioxide in Acute Promyelocytic Leukemia