Int J Stem Cells.
2020 Jul;13(2):279-286. 10.15283/ijsc20060.
Long-Term Expansion of Functional Human Pluripotent Stem Cell-Derived Hepatic Organoids
- Affiliations
-
- 1Stem Cell Convergence Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Korea
- 2Department of Functional Genomics, Korea University of Science & Technology (UST), Daejeon, Korea
- 3Biomedical Translational Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Korea
- KMID: 2504340
- DOI: http://doi.org/10.15283/ijsc20060
Abstract
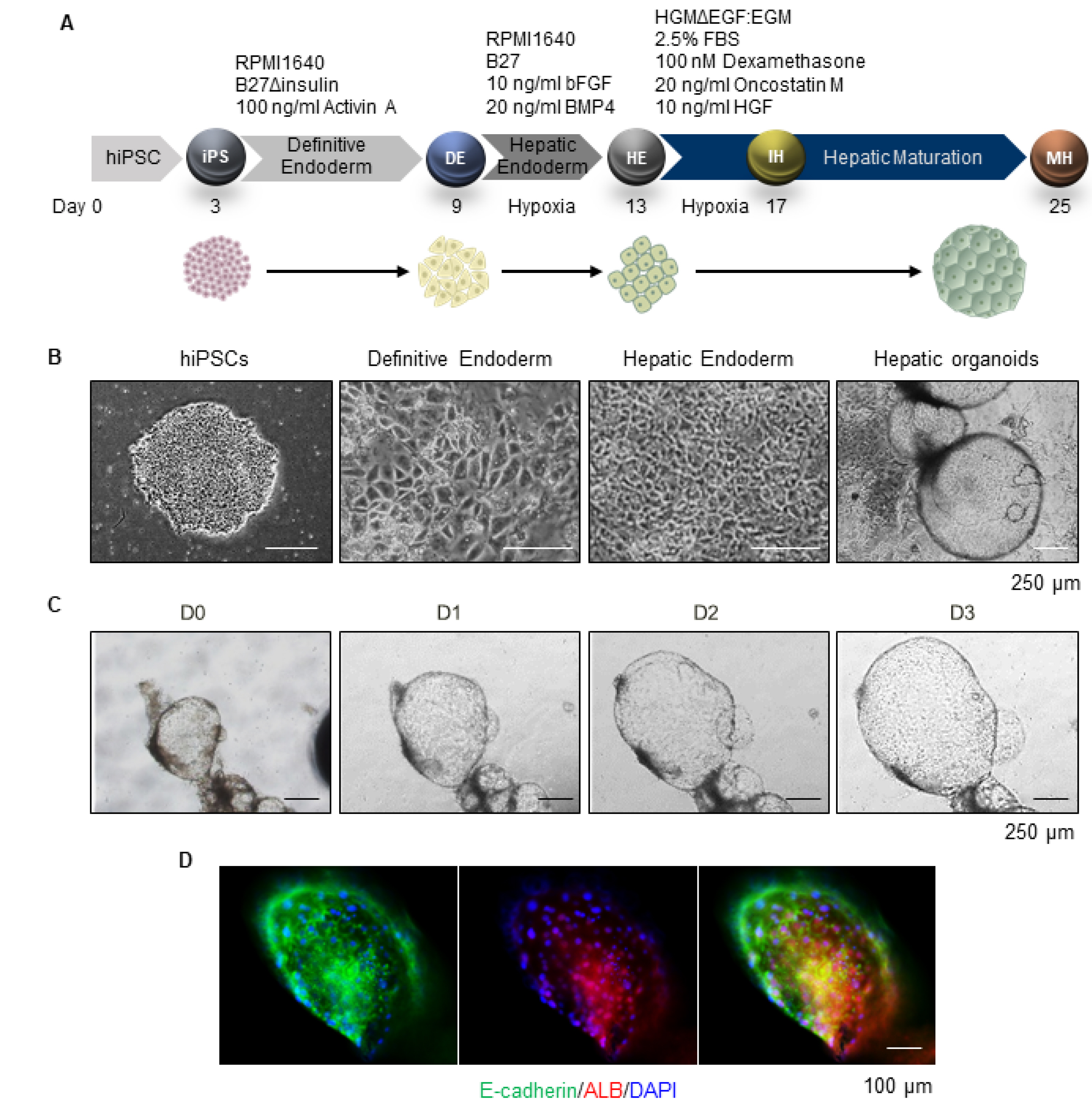
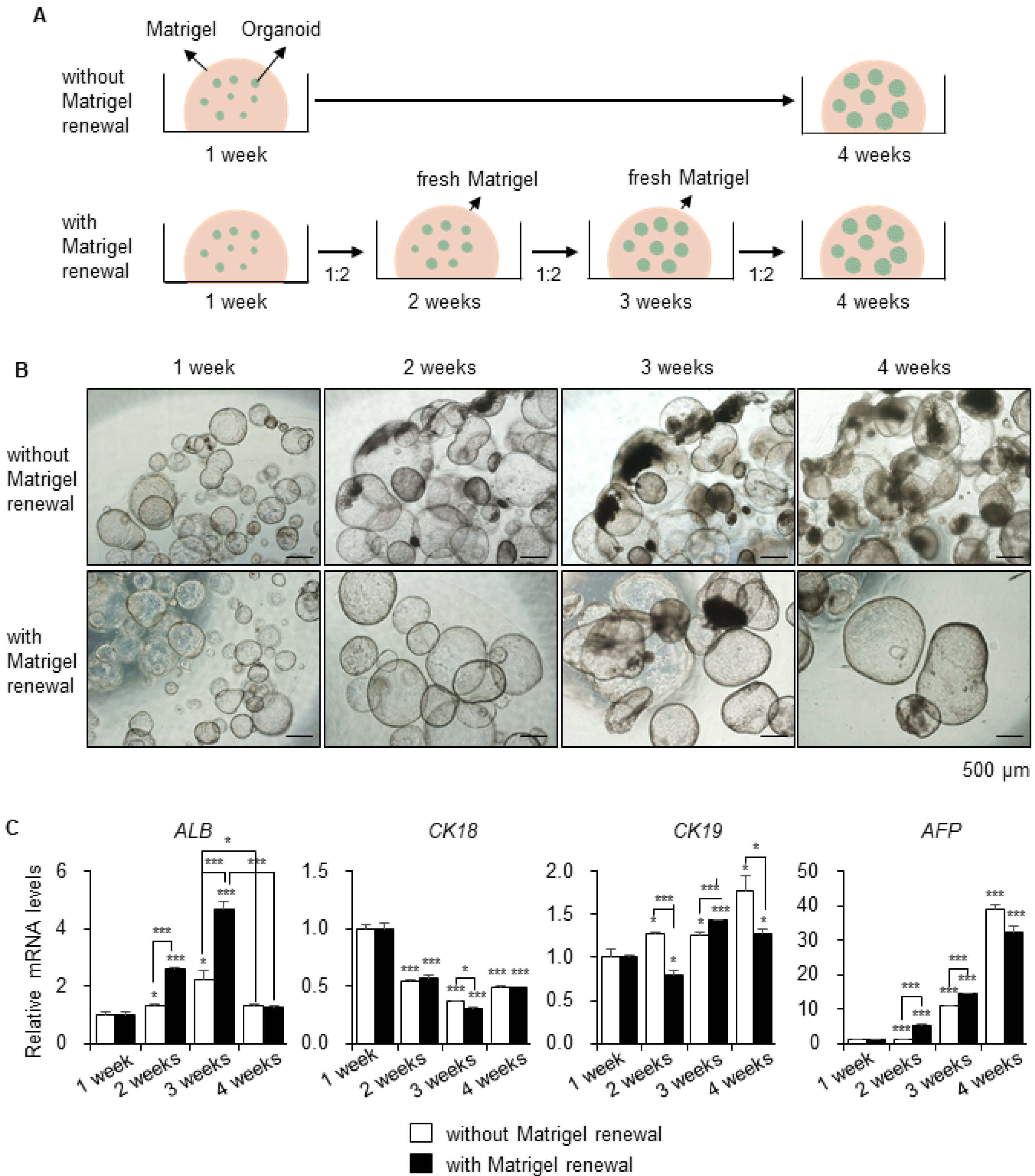
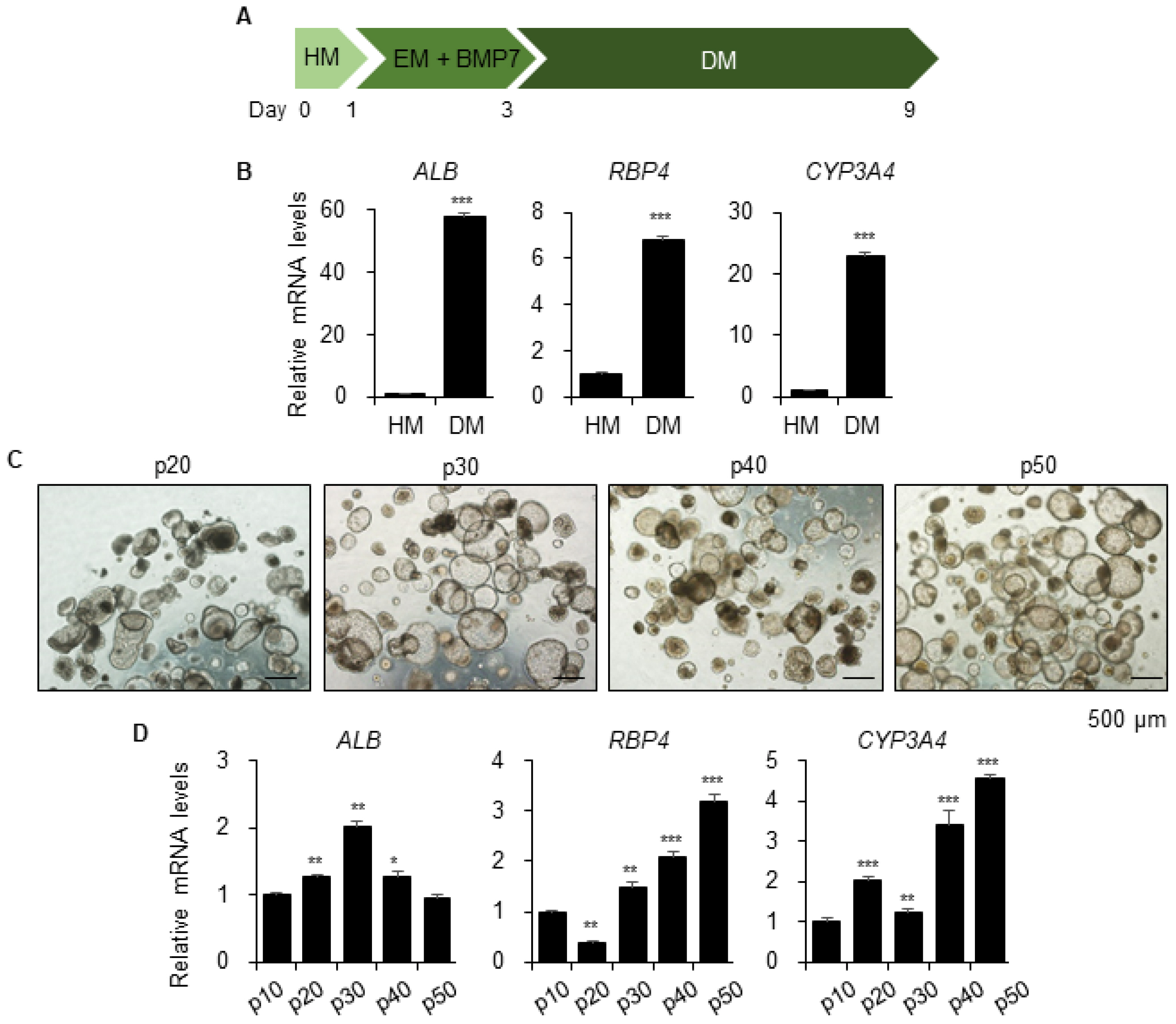
- A human cell-based liver model capable of long-term expansion and mature hepatic function is a fundamental requirement for pre-clinical drug development. We previously established self-renewing and functionally mature human pluripotent stem cell-derived liver organoids as an alternate to primary human hepatocytes. In this study, we tested long-term prolonged culture of organoids to increase their maturity. Organoid growing at the edge of Matrigel started to deteriorate two weeks after culturing, and the expression levels of the functional mature hepatocyte marker ALB were decreased at four weeks of culture. Replating the organoids weekly at a 1:2 ratio in fresh Matrigel, resulted in healthier morphology with a thicker layer compared to organoids maintained on the same Matrigel and significantly increased ALB expression until three weeks, although, it decreased sharply at four weeks. The levels of the fetal hepatocyte marker AFP were considerably increased in long-term cultures of organoids. Therefore, we performed serial passaging of organoids, whereby they were mechanically split weekly at a 1:3∼1:5 ratio in fresh Matrigel. The organoids expanded so far over passage 55, or 1 year, without growth retardation and maintained a normal karyotype after long-term cryopreservation. Differentiation potentials were maintained or increased after long-term passaging, while AFP expression considerably decreased after passaging. Therefore, these data demonstrate that organoids can be exponentially expanded by serial passaging, while maintaining long-term functional maturation potential. Thus, hepatic organoids can be a practical and renewable cell source for human cell-based and personalized 3D liver models.
Keyword
Figure
Reference
-
References
1. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. 2019; Burden of liver diseases in the world. J Hepatol. 70:151–171. DOI: 10.1016/j.jhep.2018.09.014. PMID: 30266282.
Article2. Yamaguchi T, Matsuzaki J, Katsuda T, Saito Y, Saito H, Ochiya T. 2019; Generation of functional human hepatocytes in vitro: current status and future prospects. Inflamm Regen. 39:13. DOI: 10.1186/s41232-019-0102-4. PMID: 31308858. PMCID: PMC6604181.
Article3. Underhill GH, Khetani SR. 2017; Bioengineered liver models for drug testing and cell differentiation studies. Cell Mol Gastroenterol Hepatol. 5:426–439.e1. DOI: 10.1016/j.jcmgh.2017.11.012. PMID: 29675458. PMCID: PMC5904032.
Article4. Clark M, Steger-Hartmann T. 2018; A big data approach to the concordance of the toxicity of pharmaceuticals in animals and humans. Regul Toxicol Pharmacol. 96:94–105. DOI: 10.1016/j.yrtph.2018.04.018. PMID: 29730448.
Article5. Huang P, Zhang L, Gao Y, He Z, Yao D, Wu Z, Cen J, Chen X, Liu C, Hu Y, Lai D, Hu Z, Chen L, Zhang Y, Cheng X, Ma X, Pan G, Wang X, Hui L. 2014; Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 14:370–384. DOI: 10.1016/j.stem.2014.01.003. PMID: 24582927.
Article6. Kim Y, Kang K, Lee SB, Seo D, Yoon S, Kim SJ, Jang K, Jung YK, Lee KG, Factor VM, Jeong J, Choi D. 2019; Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. J Hepatol. 70:97–107. DOI: 10.1016/j.jhep.2018.09.007. PMID: 30240598.
Article7. Touboul T, Hannan NR, Corbineau S, Martinez A, Martinet C, Branchereau S, Mainot S, Strick-Marchand H, Pedersen R, Di Santo J, Weber A, Vallier L. 2010; Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 51:1754–1765. DOI: 10.1002/hep.23506. PMID: 20301097.
Article8. Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA. 2010; Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 51:297–305. DOI: 10.1002/hep.23354. PMID: 19998274. PMCID: PMC2946078.
Article9. Sharma A, Sances S, Workman MJ, Svendsen CN. 2020; Multi-lineage human iPSC-derived platforms for disease modeling and drug discovery. Cell Stem Cell. 26:309–329. DOI: 10.1016/j.stem.2020.02.011. PMID: 32142662.
Article10. Liu C, Oikonomopoulos A, Sayed N, Wu JC. 2018; Modeling human diseases with induced pluripotent stem cells: from 2D to 3D and beyond. Development. 145:dev156166. DOI: 10.1242/dev.156166. PMID: 29519889. PMCID: PMC5868991.
Article11. Fowler JL, Ang LT, Loh KM. 2020; A critical look: challenges in differentiating human pluripotent stem cells into desired cell types and organoids. Wiley Interdiscip Rev Dev Biol. 9:e368. DOI: 10.1002/wdev.368. PMID: 31746148.
Article12. Sakabe K, Takebe T, Asai A. 2020; Organoid medicine in hepatology. Clin Liver Dis (Hoboken). 15:3–8. DOI: 10.1002/cld.855. PMID: 32104569. PMCID: PMC7041950.
Article13. Kuse Y, Taniguchi H. 2019; Present and future perspectives of using human-induced pluripotent stem cells and organoid against liver failure. Cell Transplant. 28(1 Suppl):160S–165S. DOI: 10.1177/0963689719888459. PMID: 31838891. PMCID: PMC7016460.
Article14. Li M, Izpisua Belmonte JC. 2019; Organoids - preclinical models of human disease. N Engl J Med. 380:569–579. DOI: 10.1056/NEJMra1806175. PMID: 30726695.
Article15. Akbari S, Arslan N, Senturk S, Erdal E. 2019; Next-generation liver medicine using organoid models. Front Cell Dev Biol. 7:345. DOI: 10.3389/fcell.2019.00345. PMID: 31921856. PMCID: PMC6933000.
Article16. Hu H, Gehart H, Artegiani B, LÖpez-Iglesias C, Dekkers F, Basak O, van Es J, Chuva de Sousa Lopes SM, Begthel H, Korving J, van den Born M, Zou C, Quirk C, Chiriboga L, Rice CM, Ma S, Rios A, Peters PJ, de Jong YP, Clevers H. 2018; Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. 175:1591–1606.e19. DOI: 10.1016/j.cell.2018.11.013. PMID: 30500538.
Article17. Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt J, van de Wetering M, Sasaki N, Boers SJ, Kemperman H, de Jonge J, Ijzermans JN, Nieuwenhuis EE, Hoekstra R, Strom S, Vries RR, van der Laan LJ, Cuppen E, Clevers H. 2015; Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 160:299–312. DOI: 10.1016/j.cell.2014.11.050. PMID: 25533785. PMCID: PMC4313365.
Article18. Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. 2013; Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 499:481–484. DOI: 10.1038/nature12271. PMID: 23823721.
Article19. Akbari S, Sevinç GG, Ersoy N, Basak O, Kaplan K, Sevinç K, Ozel E, Sengun B, Enustun E, Ozcimen B, Bagriyanik A, Arslan N, Önder TT, Erdal E. 2019; Robust, long-term culture of endoderm-derived hepatic organoids for disease modeling. Stem Cell Reports. 13:627–641. DOI: 10.1016/j.stemcr.2019.08.007. PMID: 31522975. PMCID: PMC6829764.
Article20. Wu F, Wu D, Ren Y, Huang Y, Feng B, Zhao N, Zhang T, Chen X, Chen S, Xu A. 2019; Generation of hepatobiliary organoids from human induced pluripotent stem cells. J Hepatol. 70:1145–1158. DOI: 10.1016/j.jhep.2018.12.028. PMID: 30630011.
Article21. Mun SJ, Ryu JS, Lee MO, Son YS, Oh SJ, Cho HS, Son MY, Kim DS, Kim SJ, Yoo HJ, Lee HJ, Kim J, Jung CR, Chung KS, Son MJ. 2019; Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 71:970–985. DOI: 10.1016/j.jhep.2019.06.030. PMID: 31299272.
Article22. Xia Y, Izpisua Belmonte JC. 2019; Design approaches for generating organ constructs. Cell Stem Cell. 24:877–894. DOI: 10.1016/j.stem.2019.05.016. PMID: 31173717.
Article23. Holloway EM, Capeling MM, Spence JR. 2019; Biologically inspired approaches to enhance human organoid complexity. Development. 146:dev166173. DOI: 10.1242/dev.166173. PMID: 30992275. PMCID: PMC6503984.
Article24. Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, Chang CF, Schiesser J, Aubert P, Stanley EG, Elefanty AG, Miyaoka Y, Mandegar MA, Conklin BR, Neunlist M, Brugmann SA, Helmrath MA, Wells JM. 2017; Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. 23:49–59. DOI: 10.1038/nm.4233. PMID: 27869805. PMCID: PMC5562951.
Article25. Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, Maria N, Scholvin J, Goldman M, Kinney JP, Boyden ES, Lichtman JW, Williams ZM, McCarroll SA, Arlotta P. 2017; Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 545:48–53. DOI: 10.1038/nature22047. PMID: 28445462. PMCID: PMC5659341.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Challenges Associated with the Use of Human Induced Pluripotent Stem Cell-Derived Organoids in Regenerative Medicine
- Vascularized Organoids: A More Complete Model
- Human Pluripotent Stem Cell-Derived Retinal Organoids: A Viable Platform for Investigating the Efficacy of Adeno-Associated Virus Gene Therapy
- Production of Functional Hepatobiliary Organoids from Human Pluripotent Stem Cells
- Region Specific Brain Organoids to Study Neurodevelopmental Disorders