J Korean Med Sci.
2020 Apr;35(16):e114. 10.3346/jkms.2020.35.e114.
Potential Utility of Therapeutic DrugMonitoring of Adalimumab in Predicting Short-Term Mucosal Healing and Histologic Remission in Pediatric Crohn's Disease Patients
- Affiliations
-
- 1Department of Pediatrics, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- 2Department of Pediatrics, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, Korea
- 3Department of Pediatrics, School of Medicine, Kyungpook National University, Daegu, Korea
- KMID: 2500203
- DOI: http://doi.org/10.3346/jkms.2020.35.e114
Abstract
- Background
Limited data exist regarding mucosal healing (MH) and therapeutic drug monitoring (TDM) in pediatric Crohn's disease (CD) patients treated with adalimumab (ADL). We aimed to investigate the associations between ADL trough levels (TLs) and MH, and between ADL TLs and histologic remission (HR) at 16 weeks from ADL treatment in pediatric CD patients.
Methods
This was a prospective study on moderate-to-severe luminal pediatric CD patients receiving ADL. Ileocolonoscopies and biopsies, as well as clinical activity assessments, laboratory examinations, including tests for ADL TLs and antibody to ADL, were performed 16 weeks after ADL initiation. MH was defined as a Simple Endoscopic Score for CD of 0. HR was defined as the complete absence of microscopic inflammation.
Results
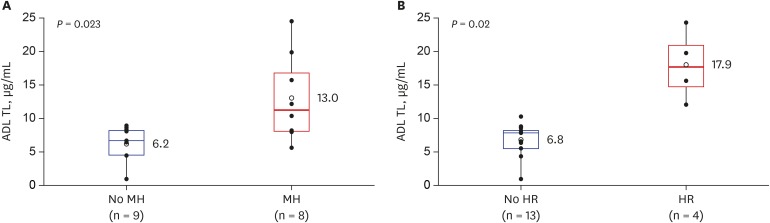
Seventeen subjects (13 males, 4 females) were included. At 16 weeks from ADL initiation, 14 (82.4%), 8 (47.1%), and 4 (23.5%) patients achieved clinical remission, MH, and HR, respectively. ADL TLs were significantly higher in patients who achieved MH compared to those who did not (13.0 ± 6.5 vs. 6.2 ± 2.6 μg/mL, respectively; P = 0.023) and also significantly higher in patients who achieved HR compared to those who did not (17.9 ± 5.3 vs. 6.8 ± 2.5 μg/mL, respectively; P = 0.02). The optimal TL for predicting MH was 8.76 μg/mL.
Conclusion
Serum ADL TLs at 16 weeks were significantly higher in pediatric patients with CD who achieved MH and HR, respectively. TDM may guide in optimizing treatment efficacy and better target MH in the era of treat-to-target.
Figure
Cited by 1 articles
-
Pediatric-onset Inflammatory Bowel Disease: What Are Different from Adult in the Treatment?
Soyoon Choi, Won Moon
Korean J Gastroenterol. 2021;77(5):220-226. doi: 10.4166/kjg.2021.067.
Reference
-
1. Pariente B, Cosnes J, Danese S, Sandborn WJ, Lewin M, Fletcher JG, et al. Development of the Crohn's disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011; 17(6):1415–1422. PMID: 21560202.
Article2. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012; 61(11):1619–1635. PMID: 22842618.
Article3. Kang B, Choe YH. Early biologic treatment in pediatric Crohn's disease: catching the therapeutic window of opportunity in early disease by treat-to-target. Pediatr Gastroenterol Hepatol Nutr. 2018; 21(1):1–11. PMID: 29383299.
Article4. Peyrin-Biroulet L, Cieza A, Sandborn WJ, Coenen M, Chowers Y, Hibi T, et al. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut. 2012; 61(2):241–247. PMID: 21646246.
Article5. Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014; 7(1):6–19. PMID: 24084775.
Article6. Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis. 2009; 15(9):1295–1301. PMID: 19340881.
Article7. Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006; 130(2):323–333. PMID: 16472588.
Article8. Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, et al. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. 2007; 56(9):1232–1239. PMID: 17299059.
Article9. Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Colombel JF, Panaccione R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007; 146(12):829–838. PMID: 17470824.10. Hyams JS, Griffiths A, Markowitz J, Baldassano RN, Faubion WA Jr, Colletti RB, et al. Safety and efficacy of adalimumab for moderate to severe Crohn's disease in children. Gastroenterology. 2012; 143(2):365–374.e2. PMID: 22562021.
Article11. Ungar B, Levy I, Yavne Y, Yavzori M, Picard O, Fudim E, et al. Optimizing anti-TNF-α therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016; 14(4):550–557.e2. PMID: 26538204.12. Yarur AJ, Rubin DT. Therapeutic drug monitoring of anti-tumor necrosis factor agents in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2015; 21(7):1709–1718. PMID: 25901974.
Article13. Yarur AJ, Jain A, Hauenstein SI, Quintero MA, Barkin JS, Deshpande AR, et al. Higher adalimumab levels are associated with histologic and endoscopic remission in patients with Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2016; 22(2):409–415. PMID: 26752470.
Article14. Zittan E, Kabakchiev B, Milgrom R, Nguyen GC, Croitoru K, Steinhart AH, et al. Higher adalimumab drug levels are associated with mucosal healing in patients with Crohn's disease. J Crohns Colitis. 2016; 10(5):510–515. PMID: 26783345.
Article15. Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol. 2006; 4(10):1248–1254. PMID: 16931170.
Article16. Van Moerkercke W, Ackaert C, Compernolle G, Jürgens M, Cleynen I, Van Assche GA, et al. 405 High infliximab trough levels are associated with mucosal healing in Crohn’s disease. Gastroenterology. 2010; 138(5):S-60.
Article17. Koga A, Matsui T, Takatsu N, Takada Y, Kishi M, Yano Y, et al. Trough level of infliximab is useful for assessing mucosal healing in Crohn's disease: a prospective cohort study. Intest Res. 2018; 16(2):223–232. PMID: 29743835.
Article18. Kang B, Choi SY, Choi YO, Lee SY, Baek SY, Sohn I, et al. Infliximab trough levels are associated with mucosal healing during maintenance treatment with infliximab in paediatric Crohn's disease. J Crohns Colitis. 2019; 13(2):189–197. PMID: 30452616.
Article19. Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014; 8(10):1179–1207. PMID: 24909831.
Article20. Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014; 58(6):795–806. PMID: 24231644.
Article21. Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991; 12(4):439–447. PMID: 1678008.
Article22. Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011; 17(6):1314–1321. PMID: 21560194.23. Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004; 60(4):505–512. PMID: 15472670.
Article24. Chiu YL, Rubin DT, Vermeire S, Louis E, Robinson AM, Lomax KG, et al. Serum adalimumab concentration and clinical remission in patients with Crohn's disease. Inflamm Bowel Dis. 2013; 19(6):1112–1122. PMID: 23584130.
Article25. Mazor Y, Almog R, Kopylov U, Ben Hur D, Blatt A, Dahan A, et al. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn's disease. Aliment Pharmacol Ther. 2014; 40(6):620–628. PMID: 25039584.
Article26. Sharma S, Eckert D, Hyams JS, Mensing S, Thakkar RB, Robinson AM, et al. Pharmacokinetics and exposure-efficacy relationship of adalimumab in pediatric patients with moderate to severe Crohn's disease: results from a randomized, multicenter, phase-3 study. Inflamm Bowel Dis. 2015; 21(4):783–792. PMID: 25723614.27. Roblin X, Marotte H, Rinaudo M, Del Tedesco E, Moreau A, Phelip JM, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014; 12(1):80–84.e2. PMID: 23891927.
Article28. Papamichael K, Baert F, Tops S, Assche GV, Rutgeerts P, Vermeire S, et al. Post-induction adalimumab concentration is associated with short-term mucosal healing in patients with ulcerative colitis. J Crohns Colitis. 2017; 11(1):53–59. PMID: 27402915.
Article29. Bouguen G, Levesque BG, Feagan BG, Kavanaugh A, Peyrin-Biroulet L, Colombel JF, et al. Treat to target: a proposed new paradigm for the management of Crohn's disease. Clin Gastroenterol Hepatol. 2015; 13(6):1042–1050.e2. PMID: 24036054.
Article30. Vande Casteele N, Ferrante M, Van Assche G, Ballet V, Compernolle G, Van Steen K, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015; 148(7):1320–1329.e3. PMID: 25724455.
Article31. Vaughn BP, Cheifetz AS. It is time to treat to trough: staying ahead of the curve in biologic testing. Clin Gastroenterol Hepatol. 2015; 13(13):2384.
Article32. Assa A, Matar M, Turner D, Broide E, Weiss B, Ledder O, et al. Proactive monitoring of adalimumab trough concentration associated with increased clinical remission in children with Crohn's disease compared with reactive monitoring. Gastroenterology. 2019; 157(4):985–996.e2. PMID: 31194979.
Article33. Jones JL, Kaplan GG, Peyrin-Biroulet L, Baidoo L, Devlin S, Melmed GY, et al. Effects of concomitant immunomodulator therapy on efficacy and safety of anti-tumor necrosis factor therapy for Crohn's disease: a meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2015; 13(13):2233–2240.e1. PMID: 26142167.
Article34. Qiu Y, Mao R, Chen BL, Zhang SH, Guo J, He Y, et al. Effects of combination therapy with immunomodulators on trough levels and antibodies against tumor necrosis factor antagonists in patients with inflammatory bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2017; 15(9):1359–1372.e6. PMID: 28232073.35. Matsumoto T, Motoya S, Watanabe K, Hisamatsu T, Nakase H, Yoshimura N, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn's disease: a prospective, randomized trial. J Crohns Colitis. 2016; 10(11):1259–1266. PMID: 27566367.
Article36. Watanabe K, Matsumoto T, Hisamatsu T, Nakase H, Motoya S, Yoshimura N, et al. Clinical and pharmacokinetic factors associated with adalimumab-induced mucosal healing in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2018; 16(4):542–549.e1. PMID: 29104132.37. Ruemmele FM, Rosh J, Faubion WA, Dubinsky MC, Turner D, Lazar A, et al. Efficacy of adalimumab for treatment of perianal fistula in children with moderately to severely active Crohn's disease: results from IMAgINE 1 and IMAgINE 2. J Crohns Colitis. 2018; 12(10):1249–1254. PMID: 29939254.
Article38. Kang B, Kim JE, Jung JH, Choe JY, Kim MJ, Choe YH, et al. Korean children and adolescents with Crohn's disease are more likely to present with perianal fistulizing disease at diagnosis compared to their european counterparts. Pediatr Gastroenterol Hepatol Nutr. 2020; 23(1):49–62. PMID: 31988875.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adalimumab Treatment in Pediatric-Onset Crohn's Disease Patients after Infliximab Failure: A Single Center Study
- Long-term safety and effectiveness of adalimumab in Japanese patients with Crohn’s disease: 3-year results from a real-world study
- Pulmonary Extraintestinal Manifestation of Crohn's Disease Treated Successfully with Adalimumab
- Efficacy of Adalimumab in Korean Patients with Crohn's Disease
- Early Biologic Treatment in Pediatric Crohn's Disease: Catching the Therapeutic Window of Opportunity in Early Disease by Treat-to-Target