Intest Res.
2021 Oct;19(4):408-418. 10.5217/ir.2020.00025.
Long-term safety and effectiveness of adalimumab in Japanese patients with Crohn’s disease: 3-year results from a real-world study
- Affiliations
-
- 1Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Japan
- 2Inflammatory Bowel Disease Center, Toho University, Sakura Medical Centre, Sakura, Japan
- 3Medical, AbbVie GK, Tokyo, Japan
- 4Center for Diagnostic and Therapeutic Endoscopy, Keio University School of Medicine, Tokyo, Japan
- 5Department of Gastroenterology, Fukuoka University Chikushi Hospital, Chikushino, Japan
- 6Advanced Research Institute, Tokyo Medical and Dental University, Tokyo, Japan
- 7Center for Advanced IBD Research and Treatment, Kitasato Institute Hospital, Kitasato University, Tokyo, Japan
- KMID: 2521604
- DOI: http://doi.org/10.5217/ir.2020.00025
Abstract
- Background/Aims
Crohn’s disease is a chronic disorder; therefore, it is essential to investigate long-term safety and efficacy of treatments. This study assessed the safety and effectiveness of adalimumab for up to 3 years in Japanese patients with Crohn’s disease in real-world settings.
Methods
This was a multicenter, single-cohort, observational study of patients with Crohn’s disease. Safety assessments included incidence of adverse drug reactions. Effectiveness assessments included clinical remission, mucosal healing, and Work Productivity and Activity Impairment (WPAI).
Results
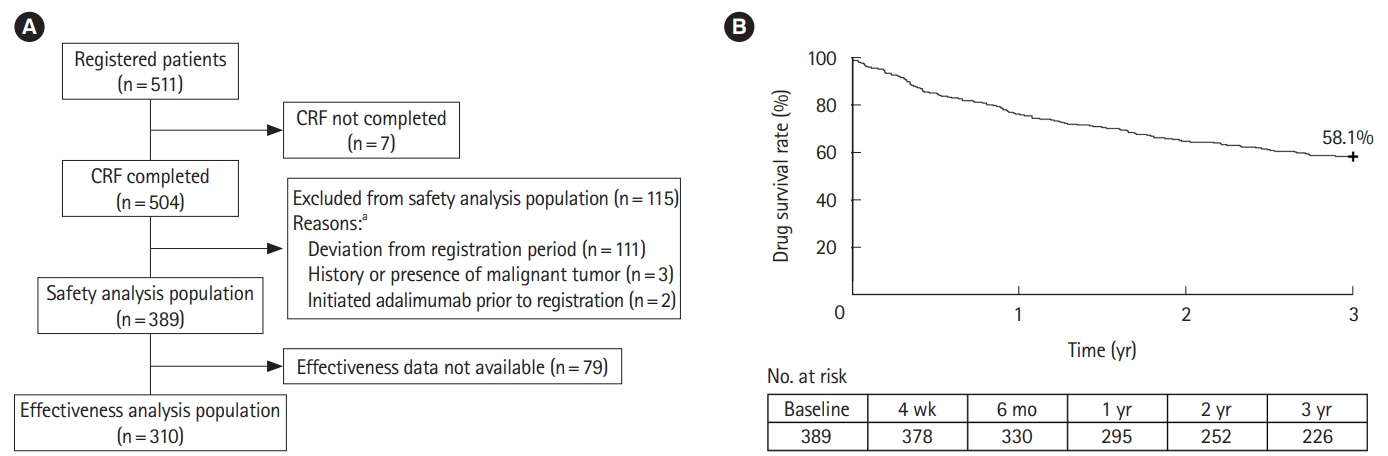
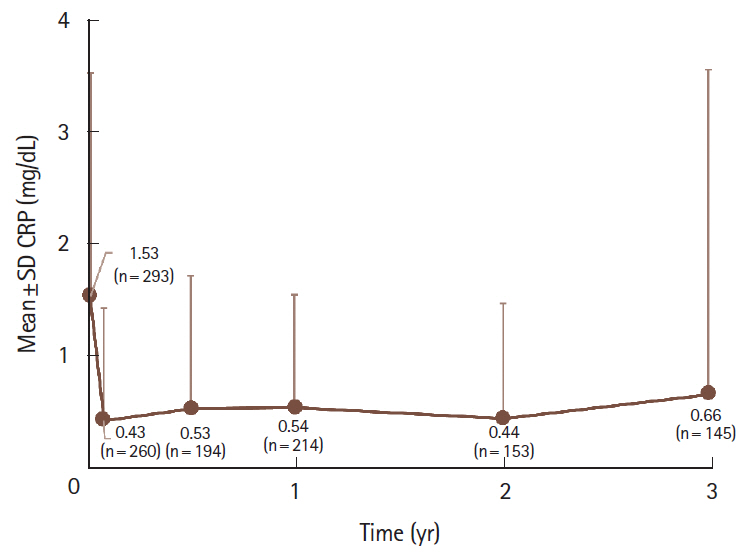
The safety and effectiveness analysis populations comprised 389 and 310 patients, respectively. Mean (standard deviation) exposure to adalimumab in the safety analysis population was 793.4 (402.8) days, with a 58.1% retention rate. A total of 105 patients (27.0%) and 43 patients (11.1%) experienced adverse drug reactions and serious adverse drug reactions, respectively, with no patient reporting tuberculosis or hepatitis B. Infections and serious infections were reported in 37 patients (9.5%) and 17 patients (4.4%), respectively. Malignancy was reported as an adverse drug reaction in 2 patients (0.5%). Remission rate increased from 37.8% (98/259) at baseline to 73.9% (167/226) at week 4 and remained > 70% over 3 years. Proportion of patients without mucosal ulcerations increased from 2.7% (2/73) at baseline to 42.3% (11/26) between years > 2 to ≤ 3. WPAI improvement started at 4 weeks, with the overall work impairment score improving from 42.7 (n = 102) at baseline to 26.9 (n = 84) at 4 weeks.
Conclusions
Results from this study confirm the long-term safety and effectiveness of adalimumab treatment in Japanese patients with Crohn’s disease in the real-world setting.
Keyword
Figure
Cited by 3 articles
-
Prevention of postoperative recurrence in Crohn’s disease: the never-ending story
Jung-Bin Park, Sang Hyoung Park
Intest Res. 2022;20(3):279-280. doi: 10.5217/ir.2022.00081.Natural history of inflammatory bowel disease: a comparison between the East and the West
Eun Mi Song, Suk-Kyun Yang
Intest Res. 2022;20(4):418-430. doi: 10.5217/ir.2021.00104.Clinical features of enteric and colo-duodenal fistula in patients with Crohn’s disease
Jun Su Lee, Sang-Bum Kang, Kwangbeom Park, Yong Sik Yoon, Chang Sik Yu, Sung Wook Hwang, Byong Duk Ye, Suk-Kyun Yang, Jong Lyul Lee, Sang Hyoung Park
Intest Res. 2023;21(3):406-410. doi: 10.5217/ir.2022.00125.
Reference
-
1. Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012; 142:1102–1111.
Article2. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018; 113:481–517.3. Williams H, Walker D, Orchard TR. Extraintestinal manifestations of inflammatory bowel disease. Curr Gastroenterol Rep. 2008; 10:597–605.4. Zippi M, Corrado C, Pica R, et al. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World J Gastroenterol. 2014; 20:17463–17467.5. Louis E, Löfberg R, Reinisch W, et al. Adalimumab improves patient-reported outcomes and reduces indirect costs in patients with moderate to severe Crohn’s disease: results from the CARE trial. J Crohns Colitis. 2013; 7:34–43.6. Zeitz J, Ak M, Müller-Mottet S, et al. Pain in IBD patients: very frequent and frequently insufficiently taken into account. PLoS One. 2016; 11:e0156666.7. Feagan BG, Bala M, Yan S, Olson A, Hanauer S. Unemployment and disability in patients with moderately to severely active Crohn’s disease. J Clin Gastroenterol. 2005; 39:390–395.8. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018; 390:2769–2778.9. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016; 14:111–119.10. Japan Intractable Diseases Information Center. Crohn’s disease [Internet]. [cited 2019 Jul 4]. http://www.nanbyou.or.jp/entry/81.11. Murakami Y, Nishiwaki Y, Oba MS, et al. Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2014: an analysis of a nationwide survey. J Gastroenterol. 2019; 54:1070–1077.12. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007; 132:52–65.13. Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007; 56:1232–1239.14. Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007; 146:829–838.15. Panaccione R, Colombel JF, Sandborn WJ, et al. Adalimumab maintains remission of Crohn’s disease after up to 4 years of treatment: data from CHARM and ADHERE. Aliment Pharmacol Ther. 2013; 38:1236–1247.16. Colombel JF, Sandborn WJ, Reinisch W, et al. Long-term safety of adalimumab in clinical trials in adult patients with Crohn’s disease or ulcerative colitis. Aliment Pharmacol Ther. 2018; 47:219–228.17. Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013; 72:517–524.18. D’Haens G, Reinisch W, Panaccione R, et al. Lymphoma risk and overall safety profile of adalimumab in patients with Crohn’s disease with up to 6 years of follow-up in the PYRAMID registry. Am J Gastroenterol. 2018; 113:872–882.19. Watanabe M, Hibi T, Lomax KG, et al. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn’s disease. J Crohns Colitis. 2012; 6:160–173.20. Watanabe M, Hibi T, Mostafa NM, et al. Long-term safety and efficacy of adalimumab in Japanese patients with moderate to severe Crohn’s disease. J Crohns Colitis. 2014; 8:1407–1416.
Article21. Ogata H, Watanabe M, Matsui T, et al. Safety of adalimumab and predictors of adverse events in 1693 Japanese patients with Crohn’s disease. J Crohns Colitis. 2016; 10:1033–1041.22. Pharmaceuticals and Medical Devices Agency. New drugs approved in FY 2016 [Internet]. c2017 [cited 2020 Oct 16]. https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0002.html.23. Loftus EV, Reinisch W, Panaccione R, et al. Adalimumab effectiveness up to six years in adalimumab-naïve patients with Crohn’s disease: results of the PYRAMID Registry. Inflamm Bowel Dis. 2019; 25:1522–1531.
Article24. Navarra SV, Tang B, Lu L, et al. Risk of tuberculosis with antitumor necrosis factor-α therapy: substantially higher number of patients at risk in Asia. Int J Rheum Dis. 2014; 17:291–298.25. Tanaka H, Kamata N, Yamada A, et al. Long-term retention of adalimumab treatment and associated prognostic factors for 1189 patients with Crohn’s disease. J Gastroenterol Hepatol. 2018; 33:1031–1038.
Article26. Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn’s disease: a prospective, randomized trial. J Crohns Colitis. 2016; 10:1259–1266.
Article27. Hisamatsu T, Matsumoto T, Watanabe K, et al. Concerns and side effects of azathioprine during adalimumab induction and maintenance therapy for Japanese patients with Crohn’s Disease: a subanalysis of a prospective randomised clinical trial [DIAMOND Study]. J Crohns Colitis. 2019; 13:1097–1104.
Article28. Louis EJ, Reinisch W, Schwartz DA, et al. Adalimumab reduces extraintestinal manifestations in patients with Crohn’s disease: a pooled analysis of 11 clinical studies. Adv Ther. 2018; 35:563–576.29. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut. 2014; 63:88–95.30. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015; 110:1324–1338.31. MacKalski BA, Bernstein CN. New diagnostic imaging tools for inflammatory bowel disease. Gut. 2006; 55:733–741.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is adalimumab safe and effective in patients with intestinal Behcet’s disease in real-world practice?
- Adalimumab Treatment in Pediatric-Onset Crohn's Disease Patients after Infliximab Failure: A Single Center Study
- Safety and effectiveness of adalimumab in the treatment of ulcerative colitis: results from a large-scale, prospective, multicenter, observational study
- Succinate-treated macrophages attenuate dextran sodium sulfate colitis in mice
- Long-Term Efficacy of Anti-Tumor Necrosis Factor Agents in Pediatric Luminal Crohn's Disease: A Systematic Review of Real-World Evidence Studies