Pediatr Gastroenterol Hepatol Nutr.
2020 Mar;23(2):121-131. 10.5223/pghn.2020.23.2.121.
Long-Term Efficacy of Anti-Tumor Necrosis Factor Agents in Pediatric Luminal Crohn's Disease: A Systematic Review of Real-World Evidence Studies
- Affiliations
-
- 1Amsterdam University Medical Centers, Location VU Medical Centre, Amsterdam, Netherlands.
- 2Department of Paediatric Gastroenterology, Hepatology and Nutrition, University of Groningen, University Medical Centre Groningen, Groningen, Netherlands. p.f.van.rheenen@umcg.nl
- KMID: 2471621
- DOI: http://doi.org/10.5223/pghn.2020.23.2.121
Abstract
- PURPOSE
To determine the long-term efficacy of the anti-tumor necrosis factor (TNF) agents, infliximab (IFX) and adalimumab (ADA), in pediatric luminal Crohn's disease (CD) by performing a systematic literature review.
METHODS
An electronic search was performed in Medline, Embase, and the Cochrane Library from inception to September 26, 2019. Eligible studies were cohort studies with observation periods that exceeded 1 year. Studies that reported time-to-event analyses were included. Events were defined as discontinuation of anti-TNF therapy for secondary loss of response. We extracted the probabilities of continuing anti-TNF therapy 1, 2, and 3 years after initiation.
RESULTS
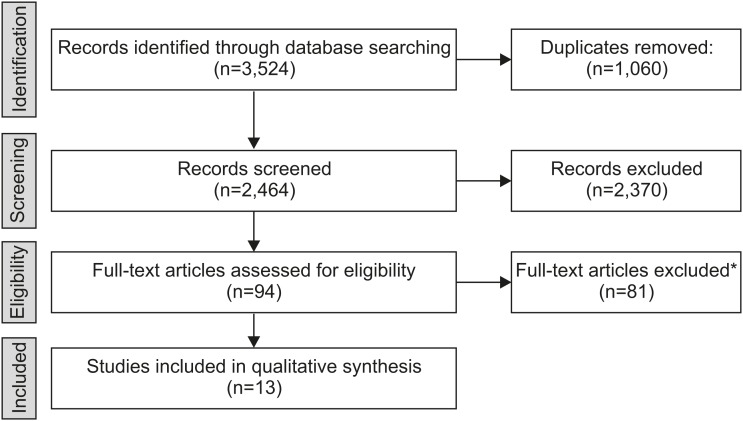
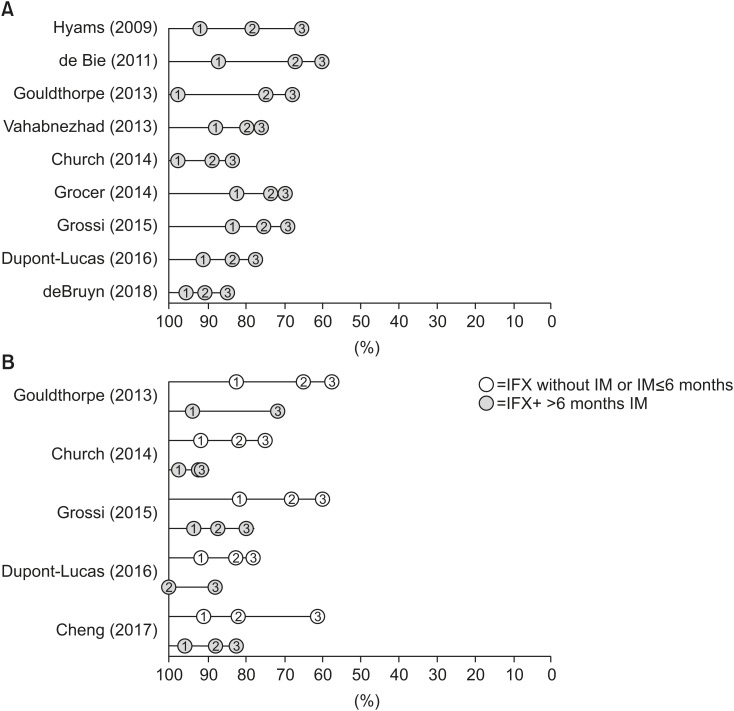
In total, 2,464 papers were screened, 94 were selected for full text review, and 13 studies (11 on IFX, 2 on ADA) met our eligibility criteria for inclusion. After 1 year, 83-97% of patients were still receiving IFX therapy. After 2 and 3 years the probability of continuing IFX therapy decreased to 67-91% and 61-85%, respectively. In total, 5 of the 11 studies subgrouped by concomitant medication consistently showed that the probabilities of continuing IFX therapy in patients with prolonged immunomodulator use were higher than those in patients on IFX monotherapy.
CONCLUSION
This review of real-world evidence studies confirms the long-term therapeutic benefit of IFX therapy in diverse cohorts of children with luminal CD. Moreover, it supports the view that combination therapy with an immunomodulator prolongs the durability of IFX therapy in patients who previously failed to recover following first-line therapy. The limited number of time-to-event studies in patients on ADA prevented us from drawing definite conclusions about its long-term efficacy.
Keyword
MeSH Terms
Figure
Reference
-
1. Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al. European Crohn's and Colitis Organisation. European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014; 8:1179–1207. PMID: 24909831.
Article2. Mack DR, Benchimol EI, Critch J, deBruyn J, Tse F, Moayyedi P, et al. Canadian Association of Gastroenterology clinical practice guideline for the medical management of pediatric luminal Crohn's disease. Gastroenterology. 2019; 157:320–348. PMID: 31320109.
Article3. Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. REACH Study Group. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007; 132:863–873. quiz 1165-6. PMID: 17324398.
Article4. Hyams JS, Griffiths A, Markowitz J, Baldassano RN, Faubion WA Jr, Colletti RB, et al. Safety and efficacy of adalimumab for moderate to severe Crohn's disease in children. Gastroenterology. 2012; 143:365–74.e2. PMID: 22562021.
Article5. Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016; 104:240–243. PMID: 27366130.
Article6. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016; 5:210. PMID: 27919275.
Article7. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007; 8:16. PMID: 17555582.
Article8. Hyams JS, Lerer T, Griffiths A, Pfefferkorn M, Kugathasan S, Evans J, et al. Pediatric Inflammatory Bowel Disease Collaborative Research Group. Long-term outcome of maintenance infliximab therapy in children with Crohn's disease. Inflamm Bowel Dis. 2009; 15:816–822. PMID: 19107783.
Article9. de Bie CI, Hummel TZ, Kindermann A, Kokke FT, Damen GM, Kneepkens CM, et al. The duration of effect of infliximab maintenance treatment in paediatric Crohn's disease is limited. Aliment Pharmacol Ther. 2011; 33:243–250. PMID: 21083595.
Article10. Gouldthorpe O, Catto-Smith AG, Alex G, Simpson D. Loss of response to long-term infliximab therapy in children with Crohn's disease. Pharmaceuticals (Basel). 2013; 6:1322–1334. PMID: 24275852.
Article11. Vahabnezhad E, Rabizadeh S, Dubinsky MC. A 10-year, single tertiary care center experience on the durability of infliximab in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2014; 20:606–613. PMID: 24552827.
Article12. Church PC, Guan J, Walters TD, Frost K, Assa A, Muise AM, et al. Infliximab maintains durable response and facilitates catch-up growth in luminal pediatric Crohn's disease. Inflamm Bowel Dis. 2014; 20:1177–1186. PMID: 24865777.
Article13. Grover Z, Biron R, Carman N, Lewindon P. Predictors of response to Infliximab in children with luminal Crohn's disease. J Crohn's Colitis. 2014; 8:739–746. PMID: 24445015.
Article14. Grossi V, Lerer T, Griffiths A, LeLeiko N, Cabrera J, Otley A, et al. Concomitant use of immunomodulators affects the durability of infliximab therapy in children with Crohn's disease. Clin Gastroenterol Hepatol. 2015; 13:1748–1756. PMID: 25911120.
Article15. Lee YM, Kang B, Lee Y, Kim MJ, Choe YH. Infliximab “top-down” strategy is superior to “step-up” in maintaining long-term remission in the treatment of pediatric crohn disease. J Pediatr Gastroenterol Nutr. 2015; 60:737–743. PMID: 25564801.
Article16. Dupont-Lucas C, Sternszus R, Ezri J, Leibovitch S, Gervais F, Amre D, et al. Identifying patients at high risk of loss of response to infliximab maintenance therapy in paediatric Crohn's disease. J Crohn's Colitis. 2016; 10:795–804. PMID: 26822611.
Article17. Cheng J, Hamilton Z, Smyth M, Barker C, Israel D, Jacobson K. Concomitant therapy with immunomodulator enhances infliximab durability in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2017; 23:1762–1773. PMID: 28837517.
Article18. deBruyn JC, Jacobson K, El-Matary W, Carroll M, Wine E, Wrobel I, et al. Long-term outcomes of infliximab use for pediatric Crohn disease: a canadian multicenter clinical practice experience. J Pediatr Gastroenterol Nutr. 2018; 66:268–273. PMID: 28657923.
Article19. Rosh JR, Lerer T, Markowitz J, Goli SR, Mamula P, Noe JD, et al. Retrospective evaluation of the safety and effect of adalimumab therapy (RESEAT) in pediatric Crohn's disease. Am J Gastroenterol. 2009; 104:3042–3049. PMID: 19724267.
Article20. Cozijnsen M, Duif V, Kokke F, Kindermann A, van Rheenen P, de Meij T, et al. Dutch PIBD Working Group Kids with Crohn and Colitis. Adalimumab therapy in children with Crohn disease previously treated with infliximab. J Pediatr Gastroenterol Nutr. 2015; 60:205–210. PMID: 25286063.
Article21. Li S, Reynaert C, Su AL, Sawh S. Efficacy and safety of infliximab in pediatric Crohn disease: a systematic review and meta-analysis. Can J Hosp Pharm. 2019; 72:227–238. PMID: 31258168.
Article22. Dziechciarz P, Horvath A, Kierkuś J. Efficacy and safety of adalimumab for paediatric Crohn's disease: a systematic review. J Crohn's Colitis. 2016; 10:1237–1244. PMID: 26995184.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preoperative use of anti-tumor necrosis factor therapy in Crohn's disease: promises and pitfalls
- Old and New Biologics and Small Molecules in Inflammatory Bowel Disease: Anti-Tumor Necrosis Factors
- Biological Therapy for Inflammatory Bowel Disease in Children
- Correlation of serum levels of anti-tumor necrosis factor agents with perianal fistula healing in Crohn’s disease: a narrative review
- Long-term clinical and real-world experience with Crohn’s disease treated with anti-tumor necrosis factor-α antibodies