Early Biologic Treatment in Pediatric Crohn's Disease: Catching the Therapeutic Window of Opportunity in Early Disease by Treat-to-Target
- Affiliations
-
- 1Department of Pediatrics, Kyungpook National University School of Medicine, Daegu, Korea.
- 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. i101016@skku.edu
- KMID: 2401767
- DOI: http://doi.org/10.5223/pghn.2018.21.1.1
Abstract
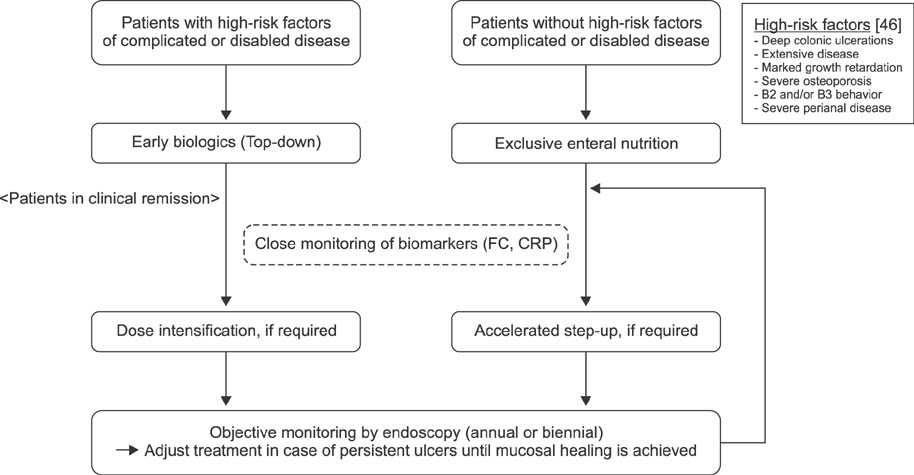
- The emergence of mucosal healing as a treatment goal that could modify the natural course of Crohn's disease and the accumulating evidence showing that biologics are most effective in achieving mucosal healing, along with the success of early treatment regimens for rheumatoid arthritis, have led to the identification of early Crohn's disease and development of the concept of catching the therapeutic window during the early disease course. Thus, an increasing number of pediatric gastroenterologists are adopting an early biologic treatment strategy with or without an immunomodulator. Although early biologic treatment is effective, cost and overtreatment are issues that limit its early use. Currently, there are insufficient data on who will benefit most from early biologics, as well as on who will not need early or even any biologics. For now, top-down biologics should be considered for patients with currently known high-risk factors of poor outcomes. For other patients, close, objective monitoring and accelerating the step-up process by means of a treat-to-target approach seems the best way to catch the therapeutic window in early pediatric Crohn's disease. The individual benefits of immunomodulator addition during early biologic treatment should be weighed against its risks and decision on early combination treatment should be made after comprehensive discussion with each patient and guardian.
MeSH Terms
Figure
Cited by 11 articles
-
Potential Utility of Therapeutic Drug Monitoring of Adalimumab in Predicting Short-Term Mucosal Healing and Histologic Remission in Pediatric Crohn's Disease Patients
So Yoon Choi, Young Ok Choi, Yon Ho Choe, Ben Kang
J Korean Med Sci. 2020;35(16):e114. doi: 10.3346/jkms.2020.35.e114.Mucosal Immunity Related to FOXP3+ Regulatory T Cells, Th17 Cells and Cytokines in Pediatric Inflammatory Bowel Disease
Jinhee Cho, Sorina Kim, Da Hee Yang, Juyeon Lee, Kyeong Won Park, Junyong Go, Chang-Lim Hyun, Youngheun Jee, Ki Soo Kang
J Korean Med Sci. 2018;33(52):. doi: 10.3346/jkms.2018.33.e336.Clinical Aspects and Treatments for Pediatric Inflammatory Bowel Diseases
Jin Soo Moon
Pediatr Gastroenterol Hepatol Nutr. 2019;22(1):50-56. doi: 10.5223/pghn.2019.22.1.50.Therapeutic Efficacy of Exclusive Enteral Nutrition with Specific Polymeric Diet in Pediatric Crohn's Disease
Yunkoo Kang, Sowon Park, Seung Kim, Sang Yong Kim, Hong Koh
Pediatr Gastroenterol Hepatol Nutr. 2019;22(1):72-79. doi: 10.5223/pghn.2019.22.1.72.Meckel's Diverticulum Diagnosed in a Child with Suspected Small Bowel Crohn's Disease
Hyun Sik Kang, Jeong Sub Lee, Chang Rim Hyun, In-Ho Jung, Ki Soo Kang
Pediatr Gastroenterol Hepatol Nutr. 2019;22(1):98-104. doi: 10.5223/pghn.2019.22.1.98.Korean Children and Adolescents with Crohn's Disease Are More Likely to Present with Perianal Fistulizing Disease at Diagnosis Compared to Their European Counterparts
Ben Kang, Jung Eun Kim, Jae Hun Jung, Jae Young Choe, Mi Jin Kim, Yon Ho Choe, Seung Kim, Hong Koh, Yoo Min Lee, Jee Hyun Lee, Yoon Lee, Ji-Hyuk Lee, Hae Jeong Lee, Hyo-Jeong Jang, Youjin Choi, So Yoon Choi, Ju Young Kim, Byung-Ho Choe
Pediatr Gastroenterol Hepatol Nutr. 2020;23(1):49-62. doi: 10.5223/pghn.2020.23.1.49.Prevention of thiopurine-induced early leukopenia in a Korean pediatric patient with Crohn’s disease who turned out to possess homozygous mutations in
NUDT15 R139C
Jaewoan Bae, Byung-Ho Choe, Ben Kang
Yeungnam Univ J Med. 2020;37(4):332-336. doi: 10.12701/yujm.2020.00178.Successful treatment with vedolizumab in an adolescent with Crohn disease who had developed active pulmonary tuberculosis while receiving infliximab
Sujin Choi, Bong Seok Choi, Byung-Ho Choe, Ben Kang
Yeungnam Univ J Med. 2021;38(3):251-257. doi: 10.12701/yujm.2020.00878.Vedolizumab Is Safe and Efficacious for the Treatment of Pediatric-Onset Inflammatory Bowel Disease Patients Who Fail a Primary Biologic Agent
Sujin Choi, Eun Sil Kim, Yiyoung Kwon, Mi Jin Kim, Yon Ho Choe, Byung-Ho Choe, Ben Kang
J Korean Med Sci. 2022;37(37):e282. doi: 10.3346/jkms.2022.37.e282.Acute pancreatitis associated with indigo naturalis in pediatric severe Crohn’s disease
Hyeon-A Kim, Hyo-rim Suh, Ben Kang, Byung-Ho Choe
Intest Res. 2019;17(1):144-148. doi: 10.5217/ir.2018.00104.Clinical aspects and treatments for pediatric inflammatory bowel diseases
Jin Soo Moon
Intest Res. 2019;17(1):17-23. doi: 10.5217/ir.2018.00139.
Reference
-
1. Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007; 369:1641–1657.
Article2. Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002; 8:244–250.
Article3. Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010; 139:1147–1155.
Article4. Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr. 2015; 169:1053–1060.
Article5. Kim S. Surgery in pediatric crohn's disease: indications, timing and post-operative management. Pediatr Gastroenterol Hepatol Nutr. 2017; 20:14–21.
Article6. Walters TD, Griffiths AM. Mechanisms of growth impairment in pediatric Crohn's disease. Nat Rev Gastroenterol Hepatol. 2009; 6:513–523.
Article7. Walters TD, Hyams JS. Can early anti-TNF-α treatment be an effective therapeutic strategy in children with Crohn's disease? Immunotherapy. 2014; 6:799–802.
Article8. Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002; 359:1541–1549.
Article9. Sands BE, Blank MA, Patel K, van Deventer SJ. ACCENT II Study. Long-term treatment of rectovaginal fistulas in Crohn's disease: response to infliximab in the ACCENT II Study. Clin Gastroenterol Hepatol. 2004; 2:912–920.
Article10. Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007; 132:863–873.
Article11. Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007; 132:52–65.
Article12. Hyams JS, Griffiths A, Markowitz J, Baldassano RN, Faubion WA Jr, Colletti RB, et al. Safety and efficacy of adalimumab for moderate to severe Crohn's disease in children. Gastroenterology. 2012; 143:365–74.e2.
Article13. Ordás I, Feagan BG, Sandborn WJ. Early use of immunosuppressives or TNF antagonists for the treatment of Crohn's disease: time for a change. Gut. 2011; 60:1754–1763.
Article14. Antunes O, Filippi J, Hébuterne X, Peyrin-Biroulet L. Treatment algorithms in Crohn's - up, down or something else? Best Pract Res Clin Gastroenterol. 2014; 28:473–483.
Article15. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012; 61:1619–1635.
Article16. Rutgeerts P, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology. 2004; 126:402–413.
Article17. Frøslie KF, Jahnsen J, Moum BA, Vatn MH. IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007; 133:412–422.
Article18. Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology. 2010; 138:463–468.
Article19. De Cruz P, Kamm MA, Prideaux L, Allen PB, Moore G. Mucosal healing in Crohn's disease: a systematic review. Inflamm Bowel Dis. 2013; 19:429–444.20. Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013; 145:996–1006.
Article21. Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010; 69:631–637.
Article22. Allen PB, Gower-Rousseau C, Danese S, Peyrin-Biroulet L. Preventing disability in inflammatory bowel disease. Therap Adv Gastroenterol. 2017; 10:865–876.
Article23. Peyrin-Biroulet L, Billioud V, D'Haens G, Panaccione R, Feagan B, Panés J, et al. Development of the Paris definition of early Crohn's disease for disease-modification trials: results of an international expert opinion process. Am J Gastroenterol. 2012; 107:1770–1776.
Article24. Pariente B, Cosnes J, Danese S, Sandborn WJ, Lewin M, Fletcher JG, et al. Development of the Crohn's disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011; 17:1415–1422.
Article25. Danese S, Fiorino G, Fernandes C, Peyrin-Biroulet L. Catching the therapeutic window of opportunity in early Crohn's disease. Curr Drug Targets. 2014; 15:1056–1063.
Article26. D'Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008; 371:660–667.27. Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 362:1383–1395.
Article28. Khanna R, Bressler B, Levesque BG, Zou G, Stitt LW, Greenberg GR, et al. Early combined immunosuppression for the management of Crohn's disease (REACT): a cluster randomised controlled trial. Lancet. 2015; 386:1825–1834.
Article29. Colombel JF, Reinisch W, Mantzaris GJ, Kornbluth A, Rutgeerts P, Tang KL, et al. Randomised clinical trial: deep remission in biologic and immunomodulator naïve patients with Crohn's disease-a SONIC post hoc analysis. Aliment Pharmacol Ther. 2015; 41:734–746.
Article30. Colombel JF, Rutgeerts PJ, Sandborn WJ, Yang M, Camez A, Pollack PF, et al. Adalimumab induces deep remission in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2014; 12:414–422.e5.
Article31. Walters TD, Kim MO, Denson LA, Griffiths AM, Dubinsky M, Markowitz J, et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-α vs an immunomodulator in children with Crohn's disease. Gastroenterology. 2014; 146:383–391.
Article32. Kang B, Choi SY, Kim HS, Kim K, Lee YM, Choe YH. Mucosal healing in paediatric patients with moderate-to-severe luminal crohn's disease under combined immunosuppression: escalation versus early treatment. J Crohns Colitis. 2016; 10:1279–1286.
Article33. Kierkus J, Dadalski M, Szymanska E, Oracz G, Wegner A, Gorczewska M, et al. The impact of infliximab induction therapy on mucosal healing and clinical remission in Polish pediatric patients with moderateto-severe Crohn's disease. Eur J Gastroenterol Hepatol. 2012; 24:495–500.
Article34. Nobile S, Gionchetti P, Rizzello F, Calabrese C, Campieri M. Mucosal healing in pediatric Crohn's disease after anti-TNF therapy: a long-term experience at a single center. Eur J Gastroenterol Hepatol. 2014; 26:458–465.35. Nuti F, Civitelli F, Bloise S, Oliva S, Aloi M, Latorre G, et al. Prospective evaluation of the achievement of mucosal healing with anti-TNF-α therapy in a paediatric Crohn's disease cohort. J Crohns Colitis. 2016; 10:5–12.
Article36. Desreumaux P, Brandt E, Gambiez L, Emilie D, Geboes K, Klein O, et al. Distinct cytokine patterns in early and chronic ileal lesions of Crohn's disease. Gastroenterology. 1997; 113:118–126.
Article37. Kugathasan S, Saubermann LJ, Smith L, Kou D, Itoh J, Binion DG, et al. Mucosal T-cell immunoregulation varies in early and late inflammatory bowel disease. Gut. 2007; 56:1696–1705.
Article38. Borrelli O, Bascietto C, Viola F, Bueno de Mesquita M, Barbato M, et al. Infliximab heals intestinal inflammatory lesions and restores growth in children with Crohn's disease. Dig Liver Dis. 2004; 36:342–347.
Article39. Crombé V, Salleron J, Savoye G, Dupas JL, Vernier-Massouille G, Lerebours E, et al. Long-term outcome of treatment with infliximab in pediatric-onset Crohn's disease: a population-based study. Inflamm Bowel Dis. 2011; 17:2144–2152.
Article40. Church PC, Guan J, Walters TD, Frost K, Assa A, Muise AM, et al. Infliximab maintains durable response and facilitates catch-up growth in luminal pediatric Crohn's disease. Inflamm Bowel Dis. 2014; 20:1177–1186.
Article41. Choi J, Kang B, Kim MJ, Sohn I, Lee HJ, Choe YH. Early infliximab yields superior long-term effects on linear growth in pediatric Crohn's disease patients. Gut Liver. 2018; DOI: 10.5009/gnl17290. [Epub ahead of print].
Article42. Lee YM, Kang B, Lee Y, Kim MJ, Choe YH. Infliximab “Top-Down” strategy is superior to “Step-Up” in maintaining long-term remission in the treatment of pediatric crohn disease. J Pediatr Gastroenterol Nutr. 2015; 60:737–743.
Article43. Wauters L, Smets F, De Greef E, Bontems P, Hoffman I, Hauser B, et al. Long-term outcomes with anti-TNF therapy and accelerated step-up in the prospective pediatric belgian crohn's disease registry (BELCRO). Inflamm Bowel Dis. 2017; 23:1584–1591.
Article44. Kugathasan S, Denson LA, Walters TD, Kim MO, Marigorta UM, Schirmer M, et al. Prediction of complicated disease course for children newly diagnosed with Crohn's disease: a multicentre inception cohort study. Lancet. 2017; 389:1710–1718.45. Kotlyar DS, Osterman MT, Diamond RH, Porter D, Blonski WC, Wasik M, et al. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011; 9:36–41.e1.
Article46. Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014; 8:1179–1207.
Article47. Kopylov U, Vutcovici M, Kezouh A, Seidman E, Bitton A, Afif W. Risk of lymphoma, colorectal and skin cancer in patients with IBD treated with immunomodulators and biologics: a quebec claims database study. Inflamm Bowel Dis. 2015; 21:1847–1853.
Article48. Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005; 54:1121–1125.
Article49. Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease: a metaanalysis. Clin Gastroenterol Hepatol. 2009; 7:874–881.
Article50. Dulai PS, Thompson KD, Blunt HB, Dubinsky MC, Siegel CA. Risks of serious infection or lymphoma with anti-tumor necrosis factor therapy for pediatric inflammatory bowel disease: a systematic review. Clin Gastroenterol Hepatol. 2014; 12:1443–1451.
Article51. Hyams JS, Dubinsky MC, Baldassano RN, Colletti RB, Cucchiara S, Escher J, et al. Infliximab is not associated with increased risk of malignancy or hemophagocytic lymphohistiocytosis in pediatric patients with inflammatory bowel disease. Gastroenterology. 2017; 152:1901–1914.e3.
Article52. Cozijnsen MA, Escher JC, Griffiths A, Turner D, de Ridder L. Benefits and risks of combining anti-tumor necrosis factor with immunomodulator therapy in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2015; 21:951–961.
Article53. Feagan BG, McDonald JW, Panaccione R, Enns RA, Bernstein CN, Ponich TP, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn's disease. Gastroenterology. 2014; 146:681–688.e1.
Article54. Toruner M, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008; 134:929–936.
Article55. Marehbian J, Arrighi HM, Hass S, Tian H, Sandborn WJ. Adverse events associated with common therapy regimens for moderate-to-severe Crohn's disease. Am J Gastroenterol. 2009; 104:2524–2533.
Article56. Day AS, Gulati AS, Patel N, Boyle B, Park KT, Saeed SA. The role of combination therapy in pediatric inflammatory bowel disease (IBD): a clinical report from the North American Society for pediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr. 2017; DOI: 10.1097/MPG.0000000000001850. [Epub ahead of print].57. Bouguen G, Levesque BG, Feagan BG, Kavanaugh A, Peyrin-Biroulet L, Colombel JF, et al. Treat to target: a proposed new paradigm for the management of Crohn's disease. Clin Gastroenterol Hepatol. 2015; 13:1042–1050.e2.
Article58. Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: special situations. J Crohns Colitis. 2010; 4:63–101.
Article59. Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn's disease. Gastroenterology. 2006; 130:650–656.
Article60. Louis E, Michel V, Hugot JP, Reenaers C, Fontaine F, Delforge M, et al. Early development of stricturing or penetrating pattern in Crohn's disease is influenced by disease location, number of flares, and smoking but not by NOD2/CARD15 genotype. Gut. 2003; 52:552–557.
Article61. Romberg-Camps MJ, Dagnelie PC, Kester AD, Hesselink-van de Kruijs MA, Cilissen M, Engels LG, et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol. 2009; 104:371–383.
Article62. Allez M, Lemann M, Bonnet J, Cattan P, Jian R, Modigliani R. Long term outcome of patients with active Crohn's disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol. 2002; 97:947–953.
Article63. Peyrin-Biroulet L, Fiorino G, Buisson A, Danese S. First-line therapy in adult Crohn's disease: who should receive anti-TNF agents? Nat Rev Gastroenterol Hepatol. 2013; 10:345–351.
Article64. Dubinsky MC, Kugathasan S, Mei L, Picornell Y, Nebel J, Wrobel I, et al. Increased immune reactivity predicts aggressive complicating Crohn's disease in children. Clin Gastroenterol Hepatol. 2008; 6:1105–1111.
Article65. Abreu MT, Taylor KD, Lin YC, Hang T, Gaiennie J, Landers CJ, et al. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn's disease. Gastroenterology. 2002; 123:679–688.
Article66. Cleynen I, González JR, Figueroa C, Franke A, McGovern D, Bortlík M, et al. Genetic factors conferring an increased susceptibility to develop Crohn's disease also influence disease phenotype: results from the IBDchip European Project. Gut. 2013; 62:1556–1565.
Article67. Siegel CA, Siegel LS, Hyams JS, Kugathasan S, Markowitz J, Rosh JR, et al. Real-time tool to display the predicted disease course and treatment response for children with Crohn's disease. Inflamm Bowel Dis. 2011; 17:30–38.
Article68. Dubinsky M. Have we changed the natural history of pediatric Crohn's disease with biologics? Dig Dis. 2014; 32:360–363.
Article69. Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015; 110:1324–1338.
Article70. Zubin G, Peter L. Predicting endoscopic crohn's disease activity before and after induction therapy in children: a comprehensive assessment of PCDAI, CRP, and fecal calprotectin. Inflamm Bowel Dis. 2015; 21:1386–1391.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Precision medicine for pediatric inflammatory bowel disease: a perspective
- Pediatric Inflammatory Bowel Disease (IBD): Phenotypic, Genetic and Therapeutic Differences between Early-Onset and Adult-Onset IBD
- Change in the treatment strategy for pediatric Crohn's disease
- Pediatric-onset Inflammatory Bowel Disease: What Are Different from Adult in the Treatment?
- Life-Threatening Lower Gastrointestinal Hemorrhage in Pediatric Crohn's Disease