Blood Res.
2020 Mar;55(1):17-26. 10.5045/br.2020.55.1.17.
Characteristics of DNMT3A mutations in acute myeloid leukemia
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. yonggoo@catholic.ac.kr, microkim@catholic.ac.kr
- 2Catholic Genetic Laboratory Center, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Cancer Research Institute, Division of Hematology, Department of Internal Medicine, Catholic Blood and Marrow Transplantation Center, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Department of Research and Development, Genetree Research, Seoul, Korea.
- KMID: 2472004
- DOI: http://doi.org/10.5045/br.2020.55.1.17
Abstract
- BACKGROUND
DNMT3A mutations occur in approximately 20% of AML cases and are associated with changes in DNA methylation. CDKN2B plays an important role in the regulation of hematopoietic progenitor cells and DNMT3A mutation is associated with CDKN2B promoter methylation. We analyzed the characteristics of DNMT3A mutations including their clinical significance in AML and their influence on promoter methylation and CDKN2B expression.
METHODS
A total of 142 adults, recently diagnosed with de novo AML, were enrolled in the study. Mutations in DNMT3A, CEBPA, and NPM1 were analyzed by bidirectional Sanger sequencing. We evaluated CDKN2B promoter methylation and expression using pyrosequencing and RT-qPCR.
RESULTS
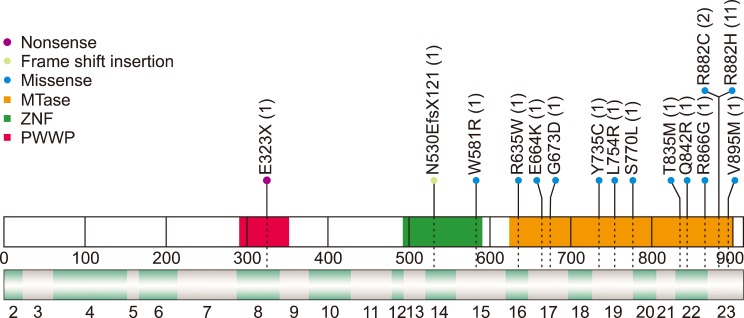
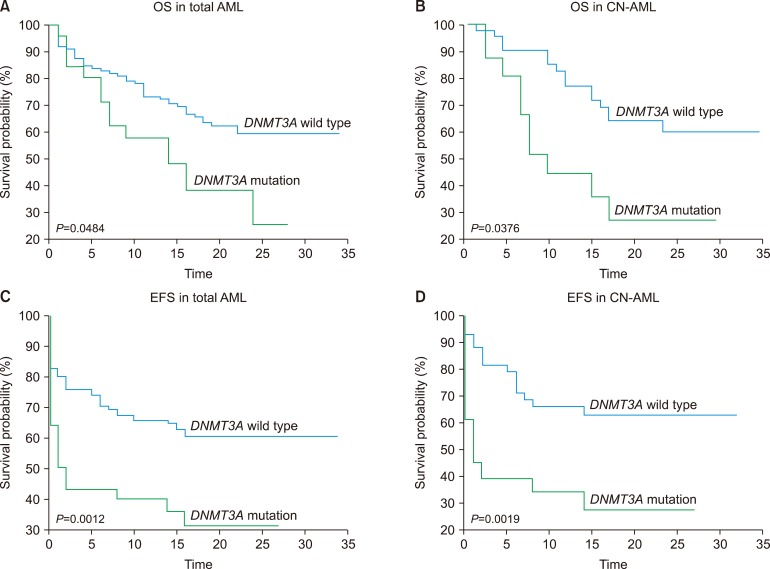
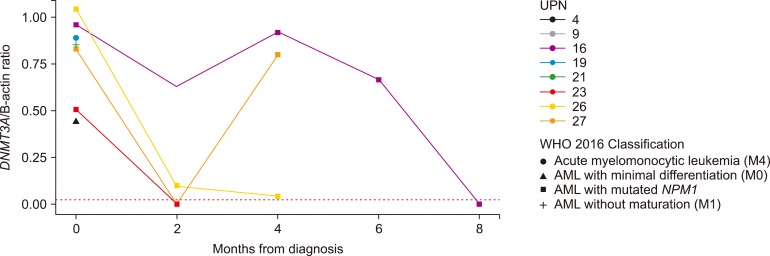
We identified DNMT3A mutations in 19.7% (N=28) of enrolled patients with AML, which increased to 29.5% when analysis was restricted to cytogenetically normal-AML. Mutations were located on exons from 8-23, and the majority, including R882, were found to be present on exon 23. We also identified a novel frameshift mutation, c.1590delC, in AML with biallelic mutation of CEBPA. There was no significant difference in CDKN2B promoter methylation according to the presence or type of DNMT3A mutations. CDKN2B expression inversely correlated with CDKN2B promoter methylation and was significantly higher in AML with R882H mutation in DNMT3A. We demonstrated that DNMT3A mutation was associated with poor AML outcomes, especially in cytogenetically normal-AML. The DNMT3A mutation remained as the independent unfavorable prognostic factor after multivariate analysis.
CONCLUSION
We characterized DNMT3A mutations in AML and revealed the association between the DNMT3A mutation and CDKN2B expression and clinical outcome.
Keyword
MeSH Terms
Figure
Reference
-
1. Betz BL, Hess JL. Acute myeloid leukemia diagnosis in the 21st century. Arch Pathol Lab Med. 2010; 134:1427–1433. PMID: 20923295.
Article2. Figueroa ME, Lugthart S, Li Y, et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell. 2010; 17:13–27. PMID: 20060365.
Article3. Delhommeau F, Dupont S, Della Valle V, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009; 360:2289–2301. PMID: 19474426.4. Ley TJ, Ding L, Walter MJ, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010; 363:2424–2433. PMID: 21067377.
Article5. Ley TJ, Miller C, Ding L, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013; 368:2059–2074. PMID: 23634996.
Article6. Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009; 361:1058–1066. PMID: 19657110.7. Jang W, Park J, Kwon A, et al. CDKN2B downregulation and other genetic characteristics in T-acute lymphoblastic leukemia. Exp Mol Med. 2019; 51:4.
Article8. Jiang Y, Dunbar A, Gondek LP, et al. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood. 2009; 113:1315–1325. PMID: 18832655.
Article9. Hájková H, Marková J, Haškovec C, et al. Decreased DNA methylation in acute myeloid leukemia patients with DNMT3A mutations and prognostic implications of DNA methylation. Leuk Res. 2012; 36:1128–1133. PMID: 22749068.
Article10. Qu Y, Lennartsson A, Gaidzik VI, et al. Differential methylation in CN-AML preferentially targets non-CGI regions and is dictated by DNMT3A mutational status and associated with predominant hypomethylation of HOX genes. Epigenetics. 2014; 9:1108–1119. PMID: 24866170.11. Im AP, Sehgal AR, Carroll MP, et al. DNMT3A and IDH mutations in acute myeloid leukemia and other myeloid malignancies: associations with prognosis and potential treatment strategies. Leukemia. 2014; 28:1774–1783. PMID: 24699305.
Article12. Yan XJ, Xu J, Gu ZH, et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat Genet. 2011; 43:309–315. PMID: 21399634.13. Russler-Germain DA, Spencer DH, Young MA, et al. The R882H DNMT3A mutation associated with AML dominantly inhibits wild-type DNMT3A by blocking its ability to form active tetramers. Cancer Cell. 2014; 25:442–454. PMID: 24656771.
Article14. De Braekeleer M, Douet-Guilbert N, De Braekeleer E. Prognostic impact of p15 gene aberrations in acute leukemia. Leuk Lymphoma. 2017; 58:257–265. PMID: 27401303.15. Sandoval JE, Huang YH, Muise A, Goodell MA, Reich NO. Mutations in the DNMT3A DNA methyltransferase in acute myeloid leukemia patients cause both loss and gain of function and differential regulation by protein partners. J Biol Chem. 2019; 294:4898–4910. PMID: 30705090.
Article16. Spencer DH, Russler-Germain DA, Ketkar S, et al. CpG island hypermethylation mediated by DNMT3A is a consequence of AML progression. Cell. 2017; 168:801–816.e13. PMID: 28215704.
Article17. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127:2391–2405. PMID: 27069254.
Article18. McGowan-Jordan J, Simons A, Schmid M, editors. ISCN 2016: An International System for Human Cytogenomic Nomenclature (2016). Basel, Switzerland: S. Karger;2016.19. Kim Y, Lee GD, Park J, et al. Quantitative fragment analysis of FLT3-ITD efficiently identifying poor prognostic group with high mutant allele burden or long ITD length. Blood Cancer J. 2015; 5:e336. PMID: 26832846.
Article20. Kwon A, Kim Y, Kim M, et al. Tissue-specific differentiation potency of mesenchymal stromal cells from perinatal tissues. Sci Rep. 2016; 6:23544. PMID: 27045658.
Article21. Jiang D, Hong Q, Shen Y, et al. The diagnostic value of DNA methylation in leukemia: a systematic review and meta-analysis. PLoS One. 2014; 9:e96822. PMID: 24810788.
Article22. Ribeiro AF, Pratcorona M, Erpelinck-Verschueren C, et al. Mutant DNMT3A: a marker of poor prognosis in acute myeloid leukemia. Blood. 2012; 119:5824–5831. PMID: 22490330.
Article23. Hou HA, Kuo YY, Liu CY, et al. DNMT3A mutations in acute myeloid leukemia: stability during disease evolution and clinical implications. Blood. 2012; 119:559–568. PMID: 22077061.
Article24. Marcucci G, Metzeler KH, Schwind S, et al. Age-related prognostic impact of different types of DNMT3A mutations in adults with primary cytogenetically normal acute myeloid leukemia. J Clin Oncol. 2012; 30:742–750. PMID: 22291079.
Article25. Brunetti L, Gundry MC, Goodell MA. DNMT3A in leukemia. Cold Spring Harb Perspect Med. 2017; 7:a030320. PMID: 28003281.
Article26. Ahn JS, Kim HJ, Kim YK, et al. DNMT3A R882 mutation with FLT3-ITD positivity is an extremely poor prognostic factor in patients with normal-karyotype acute myeloid leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016; 22:61–70. PMID: 26234722.
Article27. Tang S, Shen H, Mao X, et al. FLT3-ITD with DNMT3A R882 double mutation is a poor prognostic factor in Chinese patients with acute myeloid leukemia after chemotherapy or allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2017; 106:552–561. PMID: 28616699.
Article28. Bhatnagar B, Eisfeld AK, Nicolet D, et al. Persistence of DNMT3A R882 mutations during remission does not adversely affect outcomes of patients with acute myeloid leukaemia. Br J Haematol. 2016; 175:226–236. PMID: 27476855.29. Jeziskova I, Musilova M, Culen M, et al. Distribution of mutations in DNMT3A gene and the suitability of mutations in R882 codon for MRD monitoring in patients with AML. Int J Hematol. 2015; 102:553–557. PMID: 26290145.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Somatic Mutations Are Associated with SNP in the Progression of Individual Acute Myeloid Leukemia Patient: The Two-Hit Theory Explains Inherited Predisposition to Pathogenesis
- Myeloid Sarcoma of Peritoneum in Acute Myeloid Leukemia Patient with Inversion of Chromosome 16
- Acute Myeloid Leukemia with Intracardiac Thrombus Presenting as Acute Limb Ischemia
- A Case of Myeloid Sarcoma Preceding the Diagnosis of Acute Myeloid Leukemia
- Acute myeloid leukemia arising from chronic myelomonocytic leukemia during hypomethylating therapy