J Bacteriol Virol.
2020 Mar;50(1):17-24. 10.4167/jbv.2020.50.1.017.
Development of an Indirect ELISA Featuring Plates Coated with Column Chromatographically Purified Canine Adenovirus Type-1 Antigen
- Affiliations
-
- 1Viral Disease Research Division, Animal and Plant Quarantine Agency, MAFRA, Gimcheon, 39660, Republic of Korea. yangdk@korea.kr
- KMID: 2471834
- DOI: http://doi.org/10.4167/jbv.2020.50.1.017
Abstract
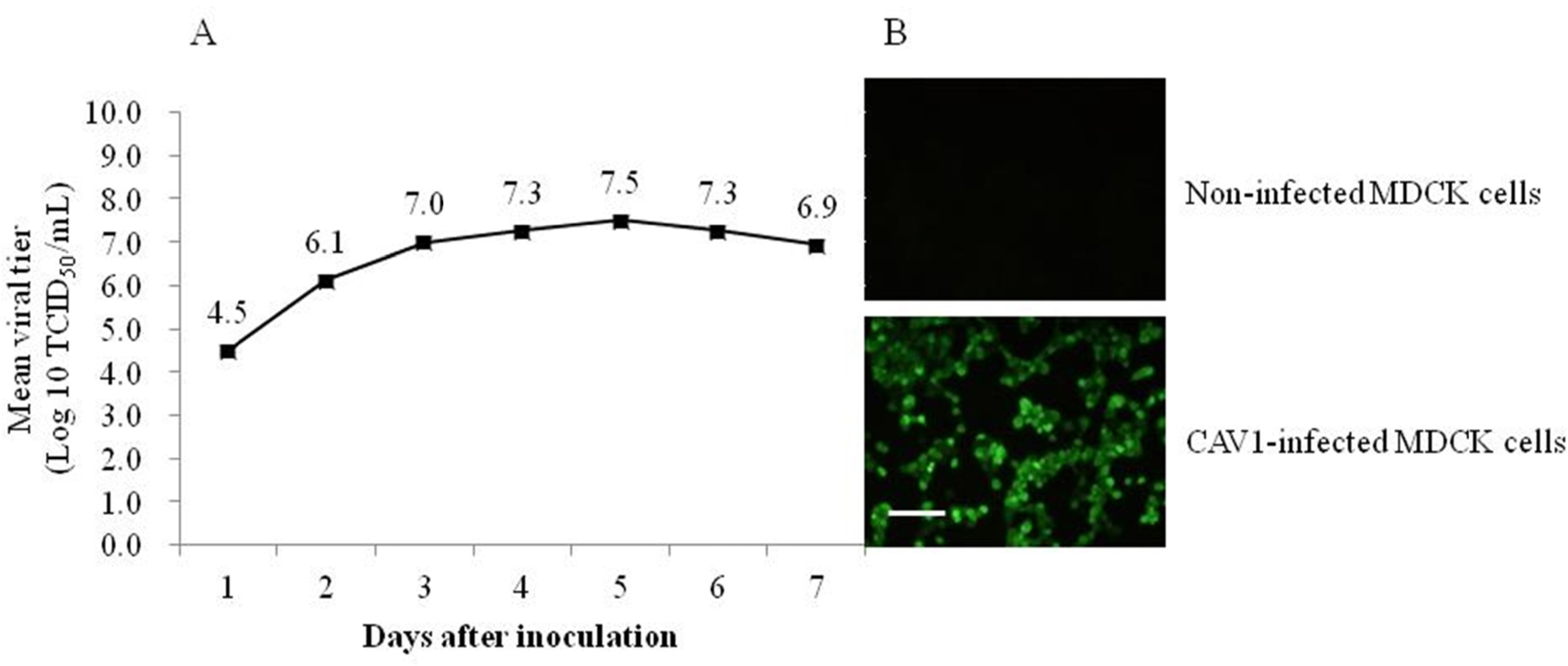
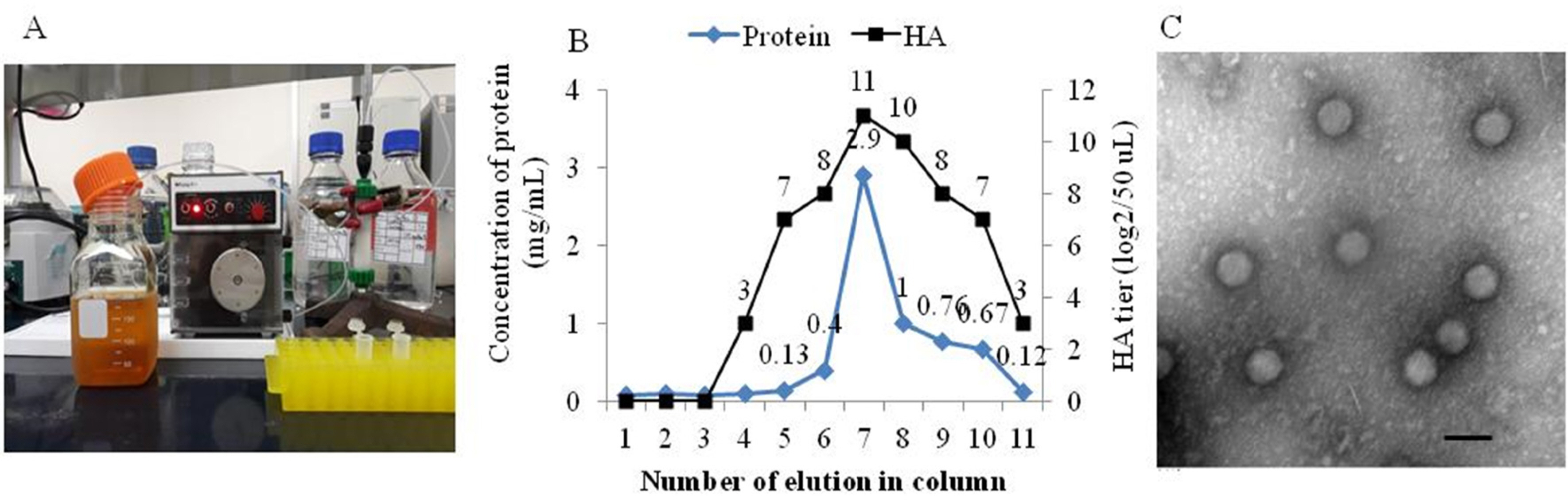
- Canine adenovirus type 1 (CAV-1) causes infectious hepatitis in members of the family Canidae, including dogs. An indirect enzyme-linked immunosorbent assay (I-ELISA) that detects CAV-1 antibodies is required for large-throughput tests of dog sera. We collected 165 serum samples from dogs of Chungbuk and Gyeongbuk provinces between February 2016 and October 2018. The Korean CAV-1 vaccine strain CAV1V was propagated in Madin-Darby canine kidney (MDCK) cells and purified via Nuvia cPrime anion-exchange chromatography; the virus served as an I-ELISA antigen. Virus-neutralizing anti-CAV-1 titers in dog sera were measured using the virus neutralization (VN) method. The I-ELISA was optimized using purified CAV-1 antigen and serum samples. This kit was used to evaluate dog sera. The VN and I-ELISA data were compared. The sensitivity, specificity, and accuracy of the I-ELISA were 97.0%, 74.2%, and 92.7% compared to the VN assay, respectively. The I-ELISA data significantly correlated with those of VN (r = 0.88). These results suggest that the I-ELISA is useful for serosurveillance of CAV-1 in dog sera.
Keyword
MeSH Terms
Figure
Reference
-
1). Hu RL, Huang G, Qiu W, Zhong ZH, Xia XZ, Yin Z. Detection and differentiation of CAV-1 and CAV-2 by polymerase chain reaction. Vet Res Commun. 2001; 25:77–84.2). Timurkan MO, Aydin H, Alkan F. Detection and molecular characterization of canine adenovirus type 2 (CAV-2) in dogs with respiratory tract symptoms in shelters in Turkey. Veterinarski arhiv. 2018; 88:467–79.3). Liu YC, Abouhaidar MG, Sira S, Campbell JB. Characterization of the genome of a vaccine strain of canine adenovirus type 1. Virus Genes. 1988; 2:69–81.
Article4). Russell WC. Adenoviruses: update on structure and function. J Gen Virol. 2009; 90:1–20.
Article5). Walker D, Abbondati E, Cox AL, Mitchell GB, Pizzi R, Sharp CP, et al. Infectious canine hepatitis in red foxes (Vulpes vulpes) in wildlife rescue centres in the UK. Vet Rec. 2016; 178:421.6). Buonavoglia C, Martella V. Canine respiratory viruses. Vet Res. 2007; 38:355–73.
Article7). Choi JW, Lee HK, Kim SH, Kim YH, Lee KK, Lee MH, et al. Canine adenovirus type 1 in a fennec fox (Vulpes zerda). J Zoo Wildl Med. 2014; 45:947–50.
Article8). Cabasso VJ. Infectious canine hepatitis virus. Ann N Y Acad Sci. 1962; 101:498–514.9). Wong M, Woolford L, Hasan NH, Hemmatzadeh F. A Novel Recombinant Canine Adenovirus Type 1 Detected from Acute Lethal Cases of Infectious Canine Hepatitis. Viral Immunol. 2017; 30:258–63.
Article10). Yang DK, Kim HH, Yoon SS, Ji M, Cho IS. Incidence and Sero-survey of Canine Adenovirus Type 2 in Various Animal Species. J Bacteriol Virol. 2018; 48:102–8.
Article11). Marusyk RG, Yamamoto T. Characterization of a canine adenovirus hemagglutinin. Can J Microbiol. 1971; 17:151–5.
Article12). Noon KF, Rogul M, Binn LN, Keefe TJ, Marchwicki RH, Thomas R, et al. An enzyme-linked immunosorbent assay for the detection of canine antibodies to canine adenoviruses. Lab Anim Sci. 1979; 29:603–9.13). Segura MM, Puig M, Monfar M, Chilló n M. Chromatography purification of canine adenoviral vectors. Hum Gene Ther Methods. 2012; 23:182–97.
Article14). Coronel J, Behrendt I, Bü rgin T, Anderlei T, Sandig V, Reichl U, et al. Influenza A virus production in a single-use orbital shaken bioreactor with ATF or TFF perfusion systems. Vaccine. 2019; 37:7011–8.
Article15). Muhamuda K, Madhusudana SN, Ravi V. Development and evaluation of a competitive ELISA for estimation of rabies neutralizing antibodies after post-exposure rabies vaccination in humans. Int J Infect Dis. 2007; 11:441–5.
Article16). Gschwender HH, Brummund M, Lehmann-Grube F. Lymphocytic Choriomeningitis virus. I. Concentration and purification of the infectious virus. J Virol. 1975; 15:1317–22.
Article17). Schagen FH, Rademaker HJ, Rabelink MJ, van Ormondt H, Fallaux FJ, van der Eb AJ, et al. Ammonium sulphate precipitation of recombinant adenovirus from culture medium: an easy method to increase the total virus yield. Gene Ther. 2000; 7:1570–4.
Article18). Kanegae Y, Makimura M, Saito I. A simple and efficient method for purification of infectious recombinant adenovirus. Jpn J Med Sci Biol. 1994; 47:157–66.
Article19). Yang DK, Kim HH, Lee SH, Ji M, Cho IS. Indirect ELISA for the Detection of Rabies Virus Antibodies in Dog Sera. J Bacteriol Virol. 2017; 47:148–55.
Article20). Burova E, Ioffe E. Chromatographic purification of recombinant adenoviral and adeno-associated viral vectors: methods and implications. Gene Ther. 2005; 12(Suppl 1):S5–17.
Article21). Konz JO, Livingood RC, Bett AJ, Goerke AR, Laska ME, Sagar SL. Serotype specificity of adenovirus purification using anion-exchange chromatography. Hum Gene Ther. 2005; 16:1346–53.
Article22). Zhu M, Carta G. Protein adsorption equilibrium and kinetics in multimodal cation exchange resins. Adsorption. 2016; 22:165–79.
Article23). He Y, Gao D, Zhang M. Expression of the nucleoprotein gene of rabies virus for use as a diagnostic reagent. J Virol Methods. 2006; 138:147–51.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of indirect ELISA for the detection of canine adenovirus type 2 antibodies in dog sera
- Diagnosis of canine brucellosis using recombinant ribosomal protein L7/L12
- Development of a monoclonal antibody-based co-agglutination test to detect enterotoxigenic Escherichia coli isolated from diarrheic neonatal calves
- Immunogenicity of a new, inactivated canine adenovirus type 2 vaccine for dogs
- Cloning and Recombinant Protein Expression of VP2 Gene of Human Parvovirus B19