J Korean Ophthalmol Soc.
2020 Mar;61(3):267-273. 10.3341/jkos.2020.61.3.267.
Effects of Hydrogen Sulfide and Nitric Oxide on the Permeability of Cultured Trabecular Meshwork Cells
- Affiliations
-
- 1Department of Ophthalmology, Daegu Catholic University College of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2471767
- DOI: http://doi.org/10.3341/jkos.2020.61.3.267
Abstract
- PURPOSE
To investigate the effects of hydrogen sulfide (Hâ‚‚S) on the permeability of a cultured human trabecular meshwork cells (HTMC) monolayer and its interaction with nitric oxide (NO).
METHODS
After exposing primary cultured HTMCs to 0, 50, 100, and 500 µM sodium hydrogen sulfide (NaHS) for 6 hours, the permeabilities through the HTMC monolayer were measured using a Transwell assay with carboxyfluorescein. The production of NO and eNOS mRNA expression were assessed using the Griess assay and reverse transcription-polymerase chain reaction, respectively. In addition, 0, 1, and 10 µM NaHS and 10 µM sodium nitroprusside (SN) were co-exposed to evaluate the possible synergistic effect of Hâ‚‚S and NO.
RESULTS
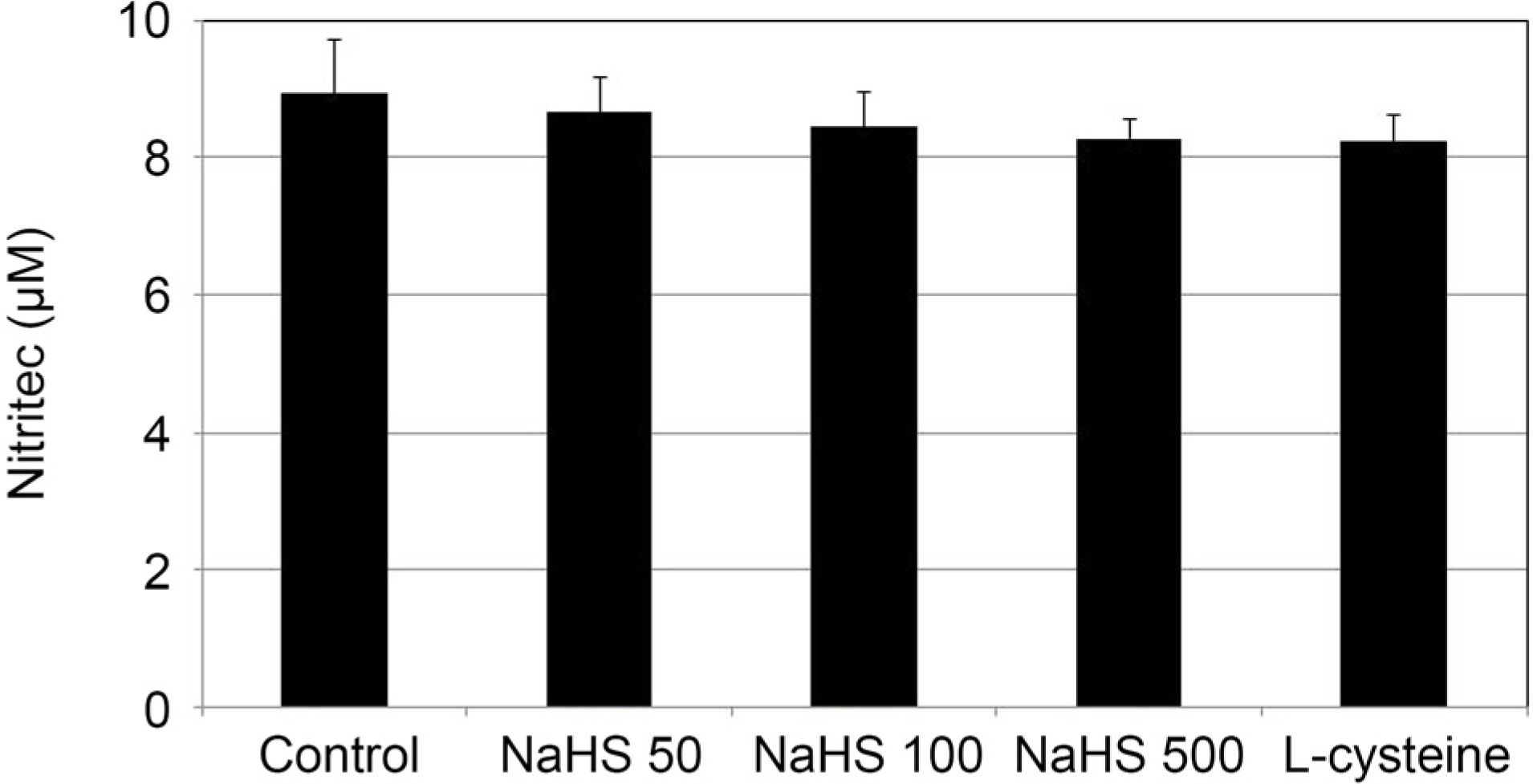
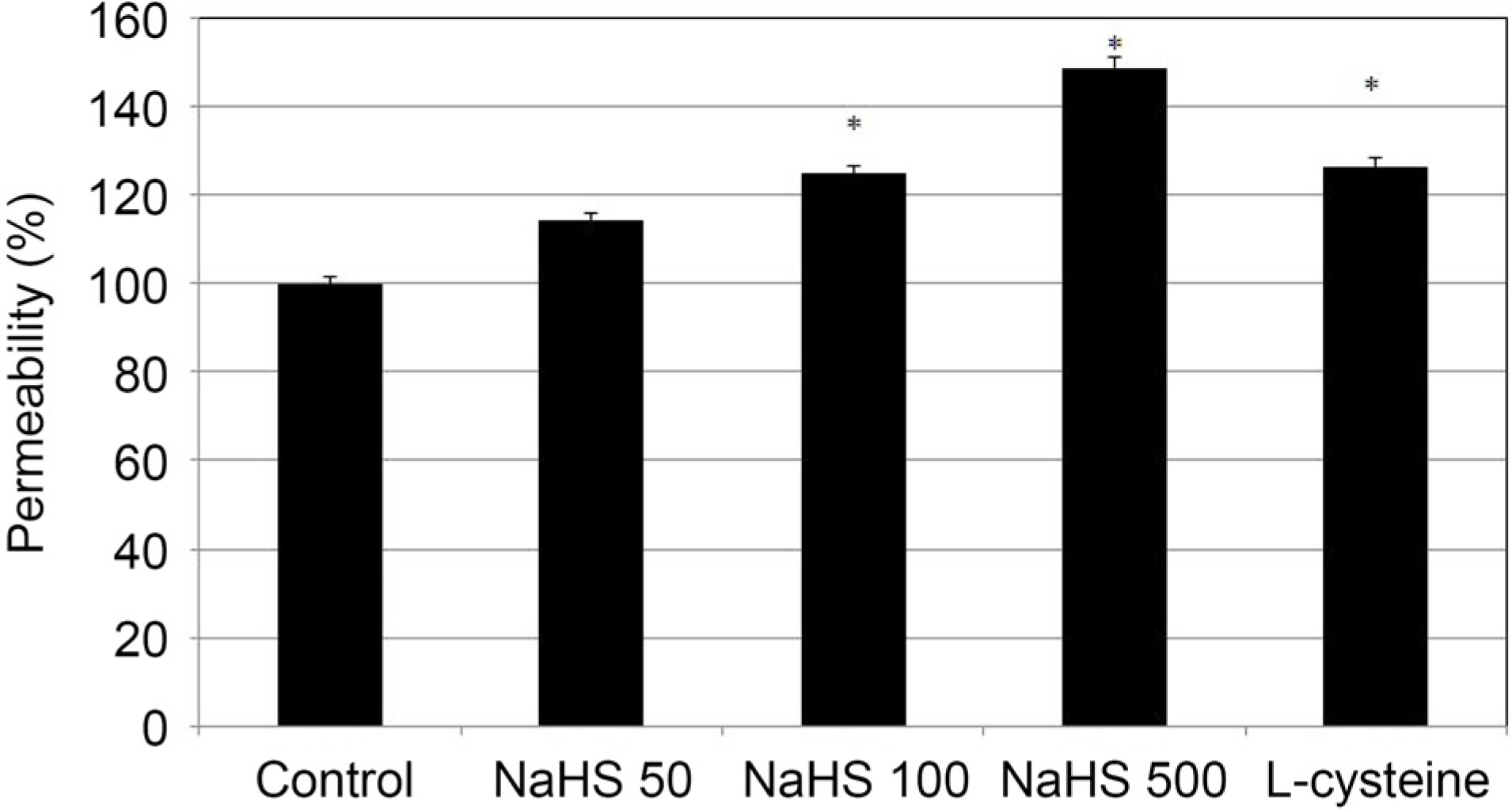
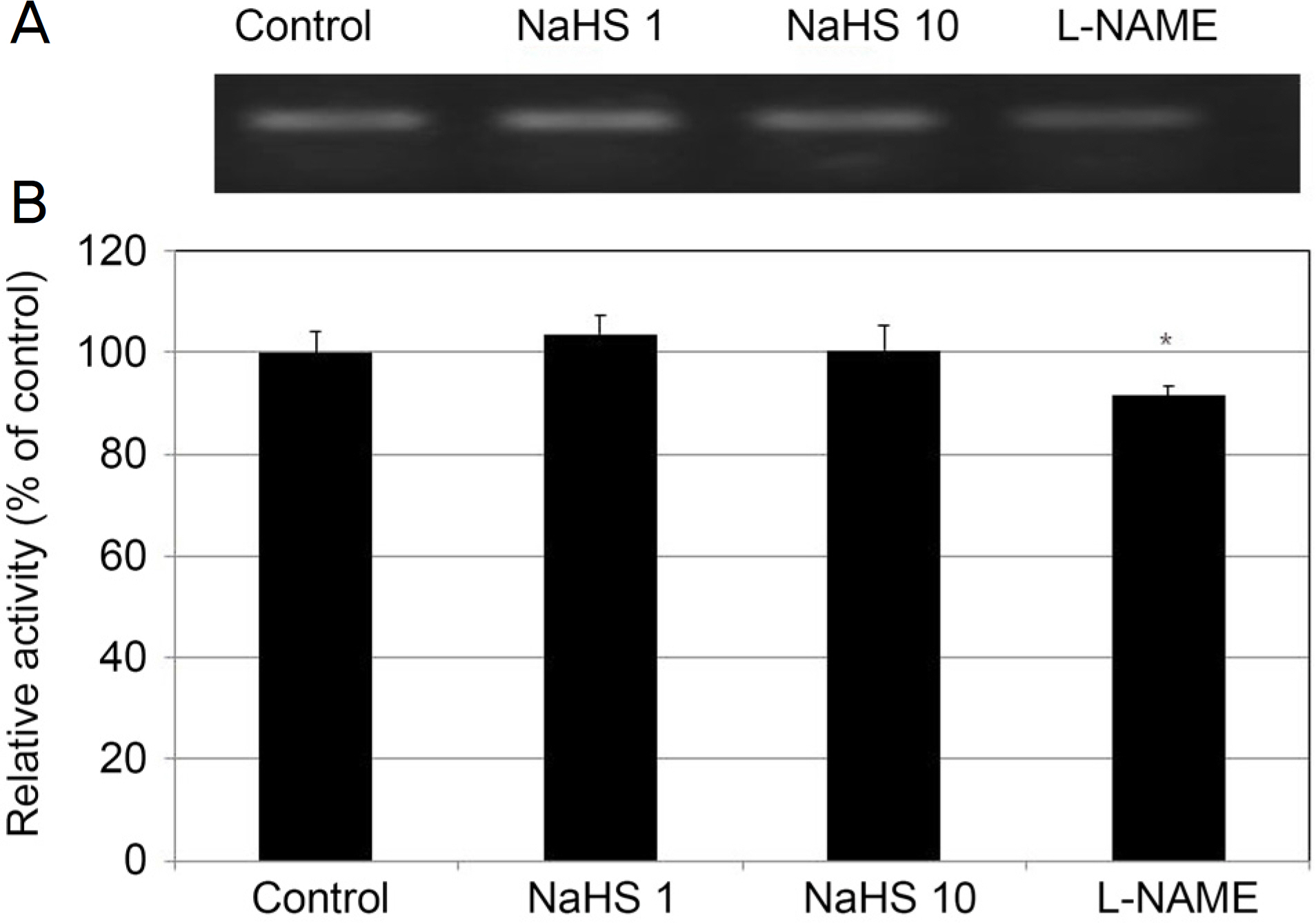
Greater than 100 µM NaHS increased the permeability through the HTMC monolayer in a dose-dependent manner (p < 0.05). These increased permeabilities were not accompanied by NO production or eNOS mRNA expression (p > 0.05). When 0, 1, and 10 µM NaHS and 10 µM SN were exposed together, there was no significant change of permeability, NO production, or eNOS mRNA expression (all, p > 0.05).
CONCLUSIONS
NaHS at high concentrations increased the permeability of the HTMC monolayer, which was not affected by NO. NaHS at low concentrations did not show a synergistic effect with NO. Thus, Hâ‚‚S at high concentrations may increase trabecular outflow, which may not be associated with NO.
MeSH Terms
Figure
Reference
-
References
1. Ł owicka E, Beł towski J. Hydrogen sulfide (H2 S) – the third gas of interest for pharmacologists. Pharmacol Rep. 2007; 59:4–24.2. Chen CQ, Xin H, Zhu YZ. Hydrogen sulfide: third gaseous trans-mitter, but with great pharmacological potential. Acta Pharmacol Sin. 2007; 28:1709–16.
Article3. Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011; 51:169–87.
Article4. Kabil O, Banerjee R. Enzymology of H2 S biogenesis, decay and signaling. Antioxid Redox Signal. 2014; 20:770–82.5. Kimura H. Production and physiological effects of hydrogen sulfide. Antioxid Redox Signal. 2014; 20:783–93.
Article5. Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide. 2014; 41:4–10.
Article6. Elsey DJ, Fowkes RC, Baxter GF. Regulation of cardiovascular cell function by hydrogen sulfide (H2 S). Cell Biochem Funct. 2010; 28:95–106.7. Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004; 18:1165–7.
Article8. Wallace JL. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol Sci. 2007; 28:501–5.
Article9. Zhang Y, Tang ZH, Ren Z, et al. Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases. Mol Cell Biol. 2013; 33:1104–13.
Article10. Kulkarni M, Njie-Mbye YF, Okpobiri I, et al. Endogenous production of hydrogen sulfide in isolated bovine eye. Neurochem Res. 2011; 36:1540–5.
Article11. Goldblum D, Mittag T. Prospects for relevant glaucoma models with retinal ganglion cell damage in the rodent eye. Vision Res. 2002; 42:471–8.
Article12. Lu M, Hu LF, Hu G, Bian JS. Hydrogen sulfide protects astrocytes against H2 O2- induced neural injury via enhancing glutamate uptake. Free Radic Biol Med. 2008; 45:1705–13.13. Sakamoto K, Suzuki Y, Kurauchi Y, et al. Hydrogen sulfide abdominal NMDA-induced neuronal injury via its anti-oxidative activity in the rat retina. Exp Eye Res. 2014; 120:90–6.14. Liu H, Anders F, Thanos S, et al. Hydrogen sulfide protects retinal ganglion cells against glaucomatous injury in vitro and in vivo. Invest Ophthalmol Vis Sci. 2017; 58:5129–41.
Article15. Huang S, Huang P, Lin Z, et al. Hydrogen sulfide supplement attenuates the apoptosis of retinal ganglion cells in experimental glaucoma. Exp Eye Res. 2018; 168:33–48.
Article16. Salvi A, Bankhele P, Jamil J, et al. Effect of hydrogen sulfide abdominals on intraocular pressure in rabbits. J Ocul Pharmacol Ther. 2016; 32:371–5.17. Robinson J, Okoro E, Ezuedu C, et al. Effects of hydrogen sul-fide-releasing compounds on aqueous humor outflow facility in porcine ocular anterior segments, Ex Vivo. J Ocul Pharmacol Ther. 2017; 33:91–7.
Article18. Kida M, Sugiyama T, Yoshimoto T, Ogawa Y. Hydrogen sulfide abdominals nitric oxide production with calcium-dependent activation of endothelial nitric oxide synthase in endothelial cells. Eur J Pharm Sci. 2013; 48:211–5.19. Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997; 237:527–31.
Article20. Bruce King S. Potential biological chemistry of hydrogen sulfide (H2 S) with the nitrogen oxides. Free Radic Biol Med. 2013; 55:1–7.21. Nagpure BV, Bian JS. Interaction of hydrogen sulfide with nitric oxide in the cardiovascular system. Oxid Med Cell Longev. 2016; 2016:6904327.
Article22. Mosmann T. Rapid colorimetric assay for cellular growth and abdominal: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.23. Fremoser FM, Jakob CA, Aebi M, Tuor U. The MTT [3-(4,5-dime-thylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl Environ Microbio. 1999; 65:3727–9.24. Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrites and nitrates in biological fluids. Analytical Biochem. 1982; 126:131–8.25. Grimes PA, Stone RA, Laties AM, Li W. Carboxyfluorescein. A probe of the blood-ocular barriers with lower membrane abdominal than fluorescein. Arch Ophthalmol. 1982; 100:635–9.26. Araie M. Carboxyfluorescein. A dye for evaluating the corneal abdominal barrier function in vivo. Exp Eye Res. 1986; 42:141–50.27. Grimes PA. Carboxyfluorescein transfer across the blood-retinal barrier evaluated by quantitative fluorescence microscopy: abdominal with fluorescein. Exp Eye Res. 1988; 46:769–83.28. Nakagawa S, Usui T, Yokoo S, et al. Toxicity evaluation of abdominal drugs using stratified human cultivated corneal abdominal sheets. Invest Ophthalmol Vis Sci. 2012; 53:5154–60.29. Lei Y, Stamer WD, Wu J, Sun X. Oxidative stress impact on barrier function of porcine angular aqueous plexus cell monolayers. Invest Ophthalmol Vis Sci. 2013; 54:4827–35.
Article30. Abe K, Kimura H. The possible role of hydrogen sulfide as an abdominal neuromodulator. J Neurosci. 1996; 16:1066–71.31. Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem. 2010; 113:14–26.
Article32. Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010; 12:1111–23.
Article33. Persa C, Osmotherly K, Chao-Wei Chen K, et al. The distribution of cystathionine beta-synthase (CBS) in the eye: implication of the presence of a trans-sulfuration pathway for oxidative stress defense. Exp Eye Res. 2006; 83:817–23.34. Wiederholt M, Dörschner N, Groth J. Effect of diuretics, channel modulators, and signal interceptors on contractility of the abdominal meshwork. Ophthalmologica. 1997; 211:153–61.35. Wiederholt M, Stumpff F. The trabecular meshwork and aqueous humor reabsorption. Civan MM, editor. Current topics in membranes. The eye's aqueous Humor: from secretion to glaucoma. 1st ed.San Diego: Academic Press;1998. p. 163–202.36. Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of abdominal meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994; 35:2515–20.37. Behar-Cohen FF, Goureau O, D'Hermies F, Courtois Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest Ophthalmol Vis Sci. 1996; 37:1711–5.38. Dismuke WM, Mbadugha CC, Ellis DZ. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am J Physiol Cell Physiol. 2008; 294:C1378–86.
Article39. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO. 2001; 20:6008–16.
Article40. Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2 S in rat tissues. Can J Physiol Pharmacol. 2003; 81:848–53.41. Kubo S, Doe I, Kurokawa Y, et al. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution to dual modulation of vascular tension. Toxicology. 2007; 232:138–46.
Article42. Geng B, Cui Y, Zhao J, et al. Hydrogen sulfide downregulates the aortic L-arginine/nitric oxide pathway in rats. Am J Physiol Regul Integr Comp Physiol. 2007; 293:R1608–18.
Article43. Yong QC, Hu LF, Wang S, et al. Hydrogen sulfide interacts with nitric oxide in the heart: possible involvement of nitroxyl. Cardiovasc Res. 2010; 88:482–91.
Article44. Wu D, Hu Q, Zhu D. An update on hydrogen sulfide and nitric abdominal interactions in the cardiovascular system. Oxid Med Cell Longev. 2018; 2018:4579140.45. Roedl JB, Bleich S, Reulbach U, et al. Homocystein levels in aqueous humor and plasma of patients with primary open-angle glaucoma. J Neural Transm (Vienna). 2007; 114:445–50.46. Roedl JB, Bleich S, Reulbach U, et al. Homocysteine in tear fluid of patients with pseudoexfoliation glaucoma. J Glaucoma. 2007; 16:234–9.
Article47. Patil A, Singh S, Opere C, Dash A. Sustained-release delivery abdominal of a slow hydrogen sulfide donor, GYY 4137, for potential application in glaucoma. AAPS PharmSciTech. 2017; 18:2291–302.48. Perrino E, Uliva C, Lanzi C, et al. New prostaglandin derivative for glaucoma treatment. Bioorg Med Chem Lett. 2009; 19:1639–42.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Tetrahydrozoline on the Permeability of Trabecular Meshwork Cell Monolayer
- Effect of Mitomycin C on the Proliferation and Nitric Oxide Production in the Cultured Trabecular Meshwork Cells
- Effect of Nitric Oxide on the Permeability of Trabecular Meshwork Cell Monolayer
- Effect of High Glucose on the Production of Reactive Oxygen Species in Trabecular Meshwork Cells
- Ascorbic Acid Enhances Nitric Oxide Production in Trabecular Meshwork Cells