J Korean Assoc Oral Maxillofac Surg.
2013 Aug;39(4):193-196.
Inflammatory granuloma caused by injectable soft tissue filler (Artecoll)
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Dentistry, Kyungpook National University, Daegu, Korea. kwondk@knu.ac.kr
- 2Department of Dentistry, Keimyung University College of Medicine, Daegu, Korea.
- 3Department of Dentistry, Yeungnam University College of Medicine, Daegu, Korea.
Abstract
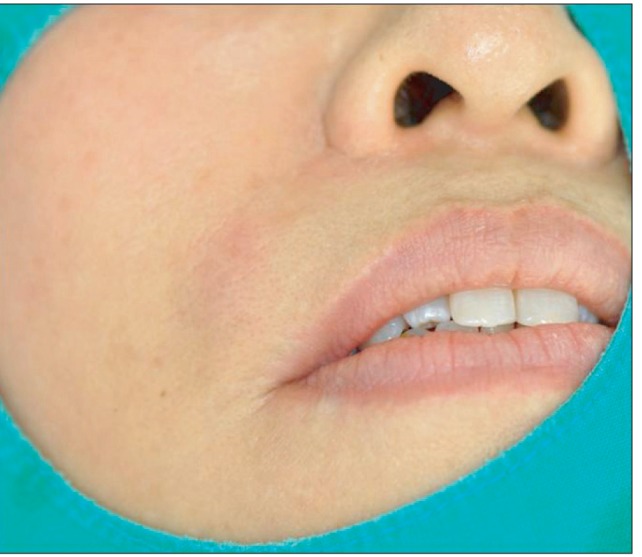
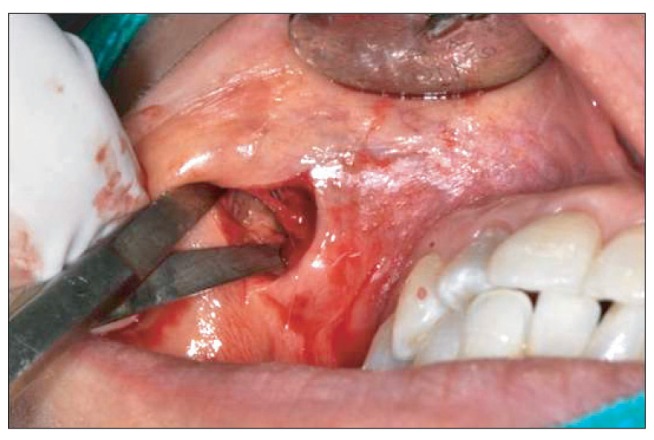
- Artecoll (Artes Medical Inc., San Diego, CA, USA) has recently been developed as a permanent synthetic cosmetic filler. We experienced an inflammatory granuloma resulting from a previous injection of Artecoll at the upper lip, which was regarded as a rare side effect of this filler. A 50-year-old female patient complained of swelling, dull pain, and heat in the right upper nasolabial fold area, which had started one week before her visit to Kyungpook National University Hospital. The patient received topical steroid therapy at a local clinic, which was not effective. At the injection site, a hard nodule was palpated and erythema was observed with mild tenderness. Antibiotic treatment and subsequent incision and drainage did not result in complete cure of the facial swelling, and the facial swelling and pain persisted. Computed tomography showed a lesion approximately 1-cm in size without clear boundaries and relatively increased nodular thickening. Finally, a subdermal lesion was removed via an intraoral vestibular approach. The lesion was diagnosed as inflammatory granuloma by a permanent biopsy. The patient had healed at two months after the filler injection. Although the soft tissue filler is widely used for cosmetic purposes, there is potential for complication, such as the inflammatory granuloma should be considered before treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. Lemperle G, Gauthier-Hazan N, Lemperle M. PMMA-Microspheres (Artecoll) for long-lasting correction of wrinkles: refinements and statistical results. Aesthetic Plast Surg. 1998; 22:356–365. PMID: 9767703.
Article2. Zimmermann US, Clerici TJ. The histological aspects of fillers complications. Semin Cutan Med Surg. 2004; 23:241–250. PMID: 15745233.
Article3. Requena C, Izquierdo MJ, Navarro M, Martínez A, Vilata JJ, Botella R, et al. Adverse reactions to injectable aesthetic microimplants. Am J Dermatopathol. 2001; 23:197–202. PMID: 11391099.
Article4. Alcalay J, Alkalay R, Gat A, Yorav S. Late-onset granulomatous reaction to Artecoll. Dermatol Surg. 2003; 29:859–862. PMID: 12859389.
Article5. Requena C, Izquierdo MJ, Navarro M, Martínez A, Vilata JJ, Botella R, et al. Adverse reactions to injectable aesthetic microimplants. Am J Dermatopathol. 2001; 23:197–202. PMID: 11391099.
Article6. Engelman DE, Bloom B, Goldberg DJ. Dermal fillers: complications and informed consent. J Cosmet Laser Ther. 2005; 7:29–32. PMID: 16020214.
Article7. Lemperle G, Hazan-Gauthier N, Lemperle M. PMMA microspheres (Artecoll) for skin and soft-tissue augmentation. Part II: clinical investigations. Plast Reconstr Surg. 1995; 96:627–634. PMID: 7638287.8. Hoffmann C, Schuller-Petrovic S, Soyer HP, Kerl H. Adverse reactions after cosmetic lip augmentation with permanent biologically inert implant materials. J Am Acad Dermatol. 1999; 40:100–102. PMID: 9922021.
Article9. Lemperle G, Romano JJ, Busso M. Soft tissue augmentation with artecoll: 10-year history, indications, techniques, and complications. Dermatol Surg. 2003; 29:573–587. PMID: 12786699.
Article10. Kim YJ, Park MY, Kim YC. Facial foreign body granuloma caused by filler (Artecoll) injection. Korean J Dermatol. 2008; 46:491–493.11. Gelfer A, Carruthers A, Carruthers J, Jang F, Bernstein SC. The natural history of polymethylmethacrylate microspheres granulomas. Dermatol Surg. 2007; 33:614–620. PMID: 17451587.
Article12. Al-Qattan MM. Late artecoll granulomas aggravated by pregnancy. Ann Plast Surg. 2007; 58:592. PMID: 17452859.
Article13. Fischer J, Metzler G, Schaller M. Cosmetic permanent fillers for soft tissue augmentation: a new contraindication for interferon therapies. Arch Dermatol. 2007; 143:507–510. PMID: 17438184.
Article14. Lemperle G, Rullan PP, Gauthier-Hazan N. Avoiding and treating dermal filler complications. Plast Reconstr Surg. 2006; 118(3 Suppl):92S–107S. PMID: 16936549.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Facial Foreign Body Granuloma Caused by Filler (Artecoll(R)) Injection
- Injectable Tissue-Engineered Soft Tissue for Tissue Augmentation
- Histologic Study of Injected Dermal Fillers (Artecoll(R), Restylane(R), Sheba(R)) in Mouse
- Treatment Algorithm of Complications after Filler Injection: Based on Wound Healing Process
- Long-Term Side Effects of Soft Tissue Filler Injection