J Korean Med Sci.
2014 Nov;29(Suppl 3):S170-S175. 10.3346/jkms.2014.29.S3.S170.
Injectable Tissue-Engineered Soft Tissue for Tissue Augmentation
- Affiliations
-
- 1Department of Plastic Surgery, Korea University College of Medicine, Seoul, Korea. pshan@kumc.or.kr
- KMID: 2151410
- DOI: http://doi.org/10.3346/jkms.2014.29.S3.S170
Abstract
- Soft tissue augmentation is a process of implanting tissues or materials to treat wrinkles or soft tissue defects in the body. Over the years, various materials have evolved to correct soft tissue defects, including a number of tissues and polymers. Autogenous dermis, autogenous fat, autogenous dermis-fat, allogenic dermis, synthetic implants, and fillers have been widely accepted for soft tissue augmentations. Tissue engineering technology has also been introduced and opened a new venue of opportunities in this field. In particular, a long-lasting filler consisting of hyaluronic acid filler and living human mesenchymal cells called "injectable tissue-engineered soft tissue" has been created and applied clinically, as this strategy has many advantages over conventional methods. Fibroblasts and adipose-derived stromal vascular fraction cells can be clinically used as injectable tissue-engineered soft tissue at present. In this review, information on the soft tissue augmentation method using the injectable tissue-engineered soft tissue is provided.
Keyword
MeSH Terms
-
Adipocytes/transplantation
Adipose Tissue/cytology
Biocompatible Materials
Connective Tissue/*surgery
Dermatologic Surgical Procedures/*methods
Face
Fibroblasts/transplantation
Humans
Hyaluronic Acid/therapeutic use
Injections, Intradermal
Mesenchymal Stem Cell Transplantation/*methods
Mesenchymal Stromal Cells
Skin
Skin Aging
Tissue Engineering/*methods
Biocompatible Materials
Hyaluronic Acid
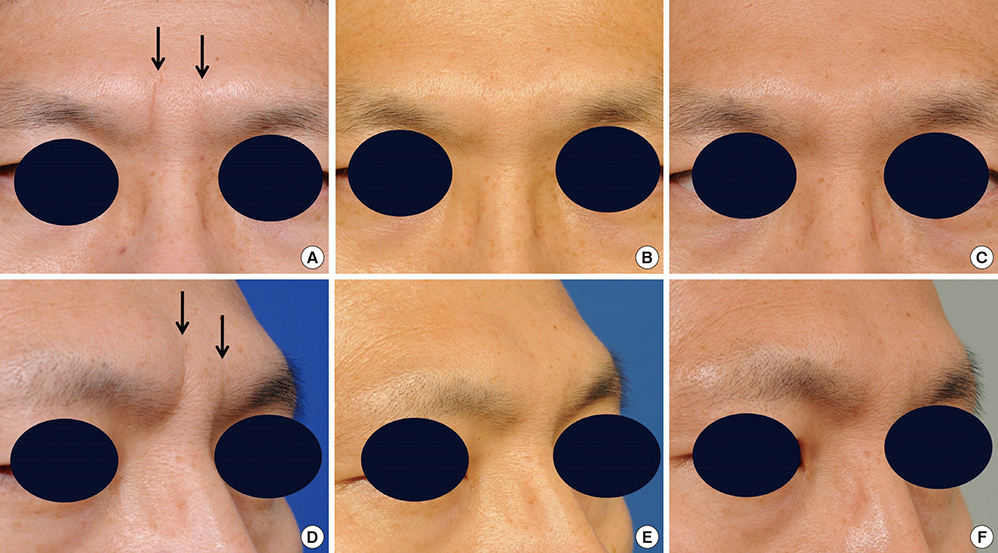
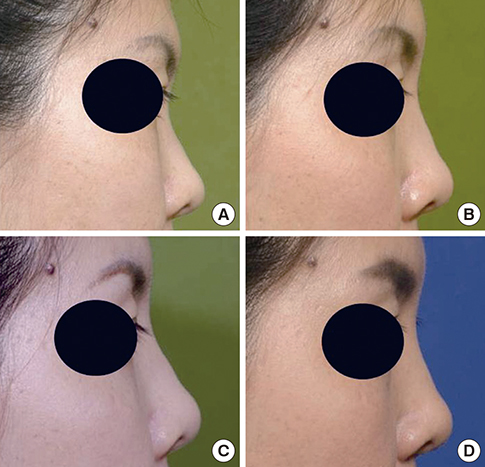
Figure
Reference
-
1. You HJ, Han SK, Lee JW, Chang H. Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes: a pilot study. Wound Repair Regen. 2012; 20:491–499.2. Han SK, You HJ. Wound coverage using advanced technology in Korea. J Korean Med Assoc. 2011; 54:594–603.3. Jeong SH, Han SK, Kim WK. Soft tissue augmentation using in vitro differentiated adipocytes: a clinical pilot study. Dermatol Surg. 2011; 37:760–767.4. Boss WK Jr, Usal H, Chernoff G, Keller GS, Lask GP, Fodor PB. Autologous cultured fibroblasts as cellular therapy in plastic surgery. Clin Plast Surg. 2000; 27:613–626.5. Lee TG, Chung S, Chung YK. A retrospective review of iatrogenic skin and soft tissue injuries. Arch Plast Surg. 2012; 39:412–416.6. Castro-Govea Y, De La Garza-Pineda O, Lara-Arias J, Chacón-Martínez H, Mecott-Rivera G, Salazar-Lozano A, Valdes-Flores E. Cell-assisted lipotransfer for the treatment of parry-romberg syndrome. Arch Plast Surg. 2012; 39:659–662.7. Lee SK, Kim DW, Dhong ES, Park SH, Yoon ES. Facial soft tissue augmentation using autologous fat mixed with stromal vascular fraction. Arch Plast Surg. 2012; 39:534–539.8. Park DK, Song I, Lee JH, You YJ. Forehead augmentation with a methyl methacrylate onlay implant using an injection-molding technique. Arch Plast Surg. 2013; 40:597–602.9. Lim EH, Sardinha JP, Myers S, Stevens M. Latent transforming growth factor-beta1 functionalised electrospun scaffolds promote human cartilage differentiation: towards an engineered cartilage construct. Arch Plast Surg. 2013; 40:676–686.10. Salibian AA, Widgerow AD, Abrouk M, Evans GR. Stem cells in plastic surgery: a review of current clinical and translational applications. Arch Plast Surg. 2013; 40:666–675.11. Graf J. Appreciating the art and science of plastic and reconstructive surgery: a year in review. Arch Plast Surg. 2013; 40:1–2.12. Fagien S, Klein AW. A brief overview and history of temporary fillers: evolution, advantages, and limitations. Plast Reconstr Surg. 2007; 120:8S–16S.13. Yoon ES, Han SK, Kim WK. Advantages of the presence of living dermal fibroblasts within restylane for soft tissue augmentation. Ann Plast Surg. 2003; 51:587–592.14. Han SK, Shin SH, Kang HJ, Kim WK. Augmentation rhinoplasty using injectable tissue-engineered soft tissue: a pilot study. Ann Plast Surg. 2006; 56:251–255.15. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13:4279–4295.16. Hong L, Peptan IA, Colpan A, Daw JL. Adipose tissue engineering by human adipose-derived stromal cells. Cells Tissues Organs. 2006; 183:133–140.17. Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008; 32:48–55.18. Lee JM, Moon KC, Han SK, Jeong SH, Kim WK. What tissue is formed after graft of adipose-derived stromal vascular fraction cells? J Craniofac Surg. 2013; 24:636–639.19. Ashjian PH, De Ugarte DA, Katz AJ, Hedrick MH. Lipoplasty: from body contouring to tissue engineering. Aesthet Surg J. 2002; 22:121–127.20. Suga H, Matsumoto D, Inoue K, Shigeura T, Eto H, Aoi N, Kato H, Abe H, Yoshimura K. Numerical measurement of viable and nonviable adipocytes and other cellular components in aspirated fat tissue. Plast Reconstr Surg. 2008; 122:103–114.21. Han SK, Kim HR, Kim WK. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: a pilot study. Wound Repair Regen. 2010; 18:342–348.22. Moon KM, Cho G, Sung HM, Jung MS, Tak KS, Jung SW, Lee HB, Suh IS. Nasal anthropometry on facial computed tomography scans for rhinoplasty in koreans. Arch Plast Surg. 2013; 40:610–615.23. Noh Y, Heo CY. The effect of phosphatidylcholine and deoxycholate compound injections to the localized adipose tissue: an experimental study with a murine model. Arch Plast Surg. 2012; 39:452–456.24. Choi J, Minn KW, Chang H. The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg. 2012; 39:585–592.25. Lee JH, Lee KH, Kim MH, Kim JP, Lee SJ, Yoon J. Possibility of undifferentiated human thigh adipose stem cells differentiating into functional hepatocytes. Arch Plast Surg. 2012; 39:593–599.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Augmentation of the Chin with a Silicone Implant
- A Case Report of Progressive Hemifacial Atrophy
- Self-inflating oral tissue expander for ridge augmentation in the severely atrophic mandible
- Strategies for Constructing Tissue-Engineered Fat for Soft Tissue Regeneration
- Microlipoinjection for Soft Tissue Augmentation