Investig Clin Urol.
2020 Mar;61(2):200-206. 10.4111/icu.2020.61.2.200.
The role of antibiotic prophylaxis in mild to moderate isolated hydronephrosis detected in antenatal screening
- Affiliations
-
- 1Department of Pediatrics, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. pornpimol.r@chula.ac.th
- KMID: 2471076
- DOI: http://doi.org/10.4111/icu.2020.61.2.200
Abstract
- PURPOSE
To determine whether continuous antibiotic prophylaxis (CAP) could prevent urinary tract infection (UTI) in mild to moderate antenatal isolated hydronephrosis (IH), characterized by hydronephrosis without ureter and bladder abnormalities, and anteroposterior renal pelvis diameter <16 mm and the Society for Fetal Urology grade <4, in neonatal renal ultrasound.
MATERIALS AND METHODS
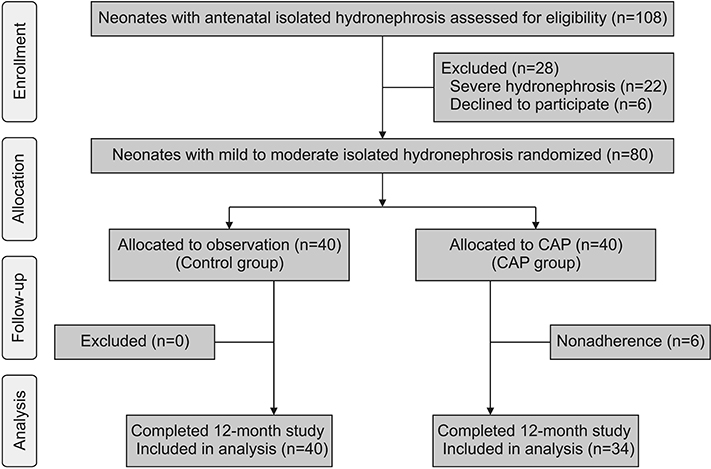
Eighty neonates aged 7 to 30 days, with antenatal hydronephrosis and mild to moderate IH on neonatal renal ultrasound, were recruited from August 2015 to December 2016. Neonates were randomly assigned to CAP until hydronephrosis resolution or aged 12 months (CAP group, n=40) or to watchful observation (control group, n=40). The primary outcome was UTI. The probability of UTI was compared between the randomized groups using the Kaplan-Meier method and the log-rank test.
RESULTS
Nonadherence occurred in 6/40 parents in the CAP arm (15.0%). Thus, only 34 patients received CAP. UTI occurred in 5/34 patients in the CAP group (14.7%) and in 4/40 controls (10.0%). The probability of UTI was increased in the CAP group (hazard ratio, 1.38; 95% confidence interval, 0.37-5.16; p=0.63). UTI caused by cotrimoxazole resistant bacteria was four times higher in the CAP group than in controls (relative risk, 4.0; 95% confidence interval, 1.2-13.5; p=0.02). The trial was prematurely terminated due to the negative impact of CAP on bacterial sensitivity.
CONCLUSIONS
The benefits of CAP in infants with mild to moderate IH were inconclusive. CAP conferred a high risk of resistant bacterial organisms when UTI occurs.
MeSH Terms
-
Anti-Bacterial Agents
Antibiotic Prophylaxis*
Arm
Bacteria
Child
Humans
Hydronephrosis*
Infant
Infant, Newborn
Kidney Pelvis
Methods
Parents
Prenatal Diagnosis*
Trimethoprim, Sulfamethoxazole Drug Combination
Ultrasonography
Ureter
Urinary Bladder
Urinary Tract Infections
Urology
Anti-Bacterial Agents
Trimethoprim, Sulfamethoxazole Drug Combination
Figure
Cited by 1 articles
-
Antenatally detected urinary tract dilatation: a pediatric nephrologist's point of view
Hyung Eun Yim
Child Kidney Dis. 2024;28(1):1-7. doi: 10.3339/ckd.24.002.
Reference
-
1. Nguyen HT, Benson CB, Bromley B, Campbell JB, Chow J, Coleman B, et al. Multidisciplinary consensus on the classification of prenatal and postnatal urinary tract dilation (UTD classification system). J Pediatr Urol. 2014; 10:982–998.
Article2. Storm DW, Braga LH, Cooper CS. Continuous antibiotic prophylaxis in pediatric urology. Urol Clin North Am. 2018; 45:525–538.
Article3. Braga LH, Mijovic H, Farrokhyar F, Pemberton J, DeMaria J, Lorenzo AJ. Antibiotic prophylaxis for urinary tract infections in antenatal hydronephrosis. Pediatrics. 2013; 131:e251–e261.
Article4. Alconcher LF, Tombesi MM. Natural history of bilateral mild isolated antenatal hydronephrosis conservatively managed. Pediatr Nephrol. 2012; 27:1119–1123.
Article5. Rianthavorn P, Limwattana S. Diagnostic accuracy of neonatal kidney ultrasound in children having antenatal hydronephrosis without ureter and bladder abnormalities. World J Urol. 2015; 33:1645–1650.
Article6. Yiee JH, Tasian GE, Copp HL. Management trends in prenatally detected hydronephrosis: national survey of pediatrician practice patterns and antibiotic use. Urology. 2011; 78:895–901.
Article7. Braga LH, Ruzhynsky V, Pemberton J, Farrokhyar F, Demaria J, Lorenzo AJ. Evaluating practice patterns in postnatal management of antenatal hydronephrosis: a national survey of Canadian pediatric urologists and nephrologists. Urology. 2014; 83:909–914.8. Merguerian PA, Herz D, McQuiston L, Van Bibber M. Variation among pediatric urologists and across 2 continents in antibiotic prophylaxis and evaluation for prenatally detected hydronephrosis: a survey of American and European pediatric urologists. J Urol. 2010; 184:4 Suppl. 1710–1715.
Article9. Zanetta VC, Rosman BM, Bromley B, Shipp TD, Chow JS, Campbell JB, et al. Variations in management of mild prenatal hydronephrosis among maternal-fetal medicine obstetricians, and pediatric urologists and radiologists. J Urol. 2012; 188:1935–1939.
Article10. Walsh TJ, Hsieh S, Grady R, Mueller BA. Antenatal hydronephrosis and the risk of pyelonephritis hospitalization during the first year of life. Urology. 2007; 69:970–974.
Article11. Sinha A, Bagga A, Krishna A, Bajpai M, Srinivas M, Uppal R, et al. Revised guidelines on management of antenatal hydronephrosis. Indian Pediatr. 2013; 50:215–231.
Article12. Subcommittee on Urinary Tract Infection. Steering Committee on Quality Improvement and Management. Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011; 128:595–610.
Article13. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012; 18:268–281.
Article14. Coelho GM, Bouzada MC, Lemos GS, Pereira AK, Lima BP, Oliveira EA. Risk factors for urinary tract infection in children with prenatal renal pelvic dilatation. J Urol. 2008; 179:284–289.
Article15. Lidefelt KJ, Herthelius M. Antenatal hydronephrosis: infants with minor postnatal dilatation do not need prophylaxis. Pediatr Nephrol. 2008; 23:2021–2024.
Article16. Easterbrook B, Capolicchio JP, Braga LH. Antibiotic prophylaxis for prevention of urinary tract infections in prenatal hydronephrosis: an updated systematic review. Can Urol Assoc J. 2017; 11:1-2Suppl1. S3–S11.
Article17. Silay MS, Undre S, Nambiar AK, Dogan HS, Kocvara R, Nijman RJM, et al. Role of antibiotic prophylaxis in antenatal hydronephrosis: a systematic review from the European Association of Urology/European Society for Paediatric Urology Guidelines Panel. J Pediatr Urol. 2017; 13:306–315.
Article18. Estrada CR, Peters CA, Retik AB, Nguyen HT. Vesicoureteral reflux and urinary tract infection in children with a history of prenatal hydronephrosis--should voiding cystourethrography be performed in cases of postnatally persistent grade II hydronephrosis? J Urol. 2009; 181:801–806. discussion 806–7.
Article19. Lee JH, Choi HS, Kim JK, Won HS, Kim KS, Moon DH, et al. Nonrefluxing neonatal hydronephrosis and the risk of urinary tract infection. J Urol. 2008; 179:1524–1528.
Article20. Shaikh N, Morone NE, Lopez J, Chianese J, Sangvai S, D'Amico F, et al. Does this child have a urinary tract infection? JAMA. 2007; 298:2895–2904.
Article21. Anderson NG, Fischer J, Leighton D, Hector-Taylor J, McEwing RL. Management in children of mild postnatal renal dilatation but without vesicoureteral reflux. Pediatr Nephrol. 2010; 25:477–483.
Article22. Dy GW, Ellison JS, Fu BC, Holt SK, Gore JL, Merguerian PA. Variable resource utilization in the prenatal and postnatal management of isolated hydronephrosis. Urology. 2017; 108:155–160.
Article23. Visuri S, Jahnukainen T, Taskinen S. Incidence of urinary tract infections in infants with antenatally diagnosed hydronephrosis-A retrospective single center study. J Pediatr Surg. 2017; 52:1503–1506.
Article24. Sencan A, Carvas F, Hekimoglu IC, Caf N, Sencan A, Chow J, et al. Urinary tract infection and vesicoureteral reflux in children with mild antenatal hydronephrosis. J Pediatr Urol. 2014; 10:1008–1013.
Article25. Vemulakonda VM, Wilcox DT, Torok MR, Hou A, Campbell JB, Kempe A. Inter-rater reliability of postnatal ultrasound interpretation in infants with congenital hydronephrosis. Int Urol Nephrol. 2015; 47:1457–1461.
Article26. Copp HL, Nelson CP, Shortliffe LD, Lai J, Saigal CS, Kennedy WA. Urologic Diseases in America Project. Compliance with antibiotic prophylaxis in children with vesicoureteral reflux: results from a national pharmacy claims database. J Urol. 2010; 183:1994–1999.
Article27. RIVUR Trial Investigators. Hoberman A, Greenfield SP, Mattoo TK, Keren R, Mathews R, et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014; 370:2367–2376.28. Nelson CP, Hoberman A, Shaikh N, Keren R, Mathews R, Greenfield SP, et al. Antimicrobial resistance and urinary tract infection recurrence. Pediatrics. 2016; 137:e20152490.
Article29. Williams G, Craig JC. Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst Rev. 2019; 4:CD001534.
Article30. Rodriguez E Jr, Weiss DA, Copp HL. Adherence to antibiotic prophylaxis in children with vesicoureteral reflux. Adv Urol. 2011; 2011:134127.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Associated Malformations and Chromosomal Defects in Antenatally Diagnosed Hydronephrosis
- Clinical Characteristics and Outcome of Isolated Fetal Hydronephrosis
- Evaluation of the Early Onset Neonatal Sepsis according to Two Antenatal Group B Streptococcus Screening Methods: Risk-Based versus Universal Screening
- The Prenatal and Postnatal Incidence of Congenital Anomalies of the Kidneys and Urinary Tract (CAKUT) Detected by Ultrasound
- Clinical study of urinary tract infection, natural courses, and prenatal ultrasonographic results according to the grades of hydronephrosis