J Lipid Atheroscler.
2020 Jan;9(1):23-49. 10.12997/jla.2020.9.1.23.
Sphingolipid Mediators of Myocardial Pathology
- Affiliations
-
- 1Department of Human and Molecular Genetics, Virginia Commonwealth University, Richmond, VA, USA.
- 2Department of Biochemistry and Molecular Biology and the Massey Cancer Center, Virginia Commonwealth University, Richmond, VA, USA. lauren.cowart@vcuhealth.org
- 3Hunter Holmes McGuire Veteran's Affairs Medical Center, Richmond, VA, USA.
- KMID: 2470799
- DOI: http://doi.org/10.12997/jla.2020.9.1.23
Abstract
- Cardiomyopathy is the leading cause of mortality worldwide. While the causes of cardiomyopathy continue to be elucidated, current evidence suggests that aberrant bioactive lipid signaling plays a crucial role as a component of cardiac pathophysiology. Sphingolipids have been implicated in the pathophysiology of cardiovascular disease, as they regulate numerous cellular processes that occur in primary and secondary cardiomyopathies. Experimental evidence gathered over the last few decades from both in vitro and in vivo model systems indicates that inhibitors of sphingolipid synthesis attenuate a variety of cardiomyopathic symptoms. In this review, we focus on various cardiomyopathies in which sphingolipids have been implicated and the potential therapeutic benefits that could be gained by targeting sphingolipid metabolism.
MeSH Terms
Figure
Reference
-
1. Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 2018; 19:175–191.
Article2. Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2010; 62:579–587.
Article3. Cremesti A, Paris F, Grassmé H, Holler N, Tschopp J, Fuks Z, et al. Ceramide enables fas to cap and kill. J Biol Chem. 2001; 276:23954–23961.
Article4. Guo W, Wong S, Xie W, Lei T, Luo Z. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am J Physiol Endocrinol Metab. 2007; 293:E576–E586.
Article5. Park TS, Yamashita H, Blaner WS, Goldberg IJ. Lipids in the heart: a source of fuel and a source of toxins. Curr Opin Lipidol. 2007; 18:277–282.
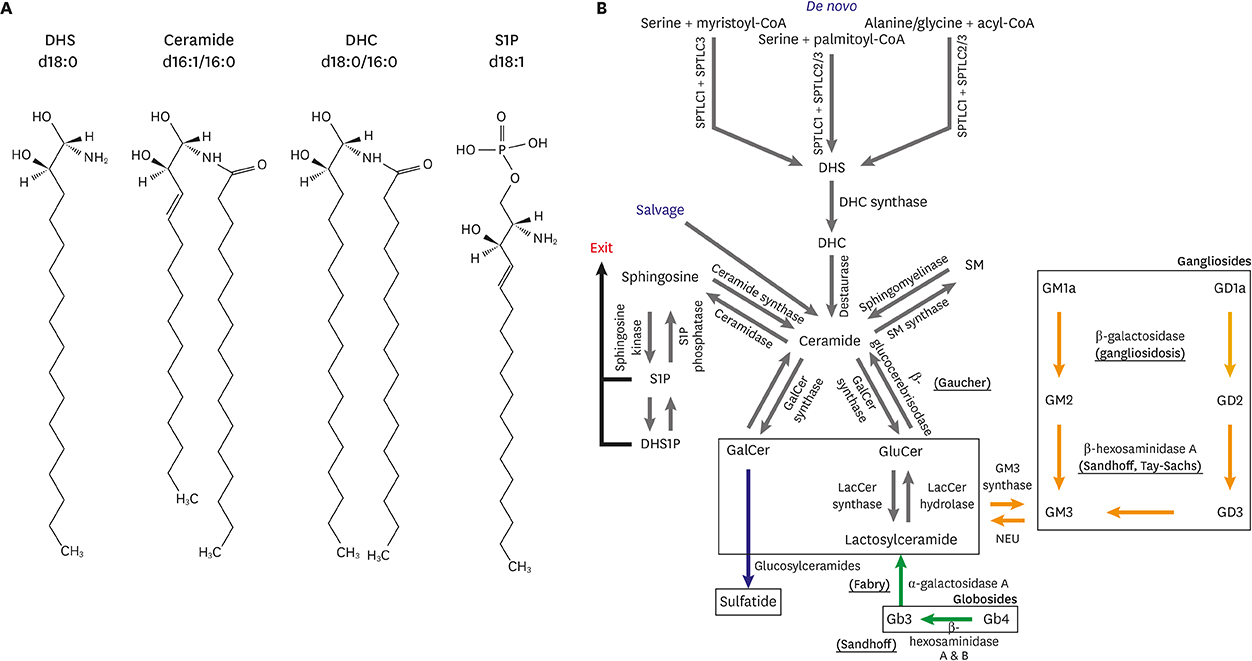
Article6. Sasset L, Zhang Y, Dunn TM, Di Lorenzo A. Sphingolipid de novo biosynthesis: a rheostat of cardiovascular homeostasis. Trends Endocrinol Metab. 2016; 27:807–819.
Article7. Hanada K, Hara T, Nishijima M. D-Serine inhibits serine palmitoyltransferase, the enzyme catalyzing the initial step of sphingolipid biosynthesis. FEBS Lett. 2000; 474:63–65.
Article8. Hannun YA, Linardic CM. Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochim Biophys Acta. 1993; 1154:223–236.
Article9. Hornemann T, Richard S, Rütti MF, Wei Y, von Eckardstein A. Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. J Biol Chem. 2006; 281:37275–37281.
Article10. Nagiec MM, Baltisberger JA, Wells GB, Lester RL, Dickson RC. The LCB2 gene of Saccharomyces and the related LCB1 gene encode subunits of serine palmitoyltransferase, the initial enzyme in sphingolipid synthesis. Proc Natl Acad Sci U S A. 1994; 91:7899–7902.
Article11. Russo SB, Tidhar R, Futerman AH, Cowart LA. Myristate-derived d16:0 sphingolipids constitute a cardiac sphingolipid pool with distinct synthetic routes and functional properties. J Biol Chem. 2013; 288:13397–13409.
Article12. Han G, Gupta SD, Gable K, Niranjanakumari S, Moitra P, Eichler F, et al. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc Natl Acad Sci U S A. 2009; 106:8186–8191.
Article13. Harmon JM, Bacikova D, Gable K, Gupta SD, Han G, Sengupta N, et al. Topological and functional characterization of the ssSPTs, small activating subunits of serine palmitoyltransferase. J Biol Chem. 2013; 288:10144–10153.
Article14. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006; 113:1807–1816.
Article15. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008; 29:270–276.
Article16. Ma RC, So WY, Tong PC, Chan JC, Cockram CS, Chow CC. Adiposity of the heart revisited: reversal of dilated cardiomyopathy in a patient with Cushing's syndrome. Int J Cardiol. 2011; 151:e22–e23.
Article17. Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008; 49:2101–2112.
Article18. Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001; 107:813–822.
Article19. Maekawa K, Hirayama A, Iwata Y, Tajima Y, Nishimaki-Mogami T, Sugawara S, et al. Global metabolomic analysis of heart tissue in a hamster model for dilated cardiomyopathy. J Mol Cell Cardiol. 2013; 59:76–85.
Article20. Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995; 11:376–381.
Article21. Zhang Y, Ikeno Y, Qi W, Chaudhuri A, Li Y, Bokov A, et al. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci. 2009; 64:1212–1220.
Article22. Cordis GA, Yoshida T, Das DK. HPTLC analysis of sphingomylein, ceramide and sphingosine in ischemic/reperfused rat heart. J Pharm Biomed Anal. 1998; 16:1189–1193.
Article23. Zhang DX, Fryer RM, Hsu AK, Zou AP, Gross GJ, Campbell WB, et al. Production and metabolism of ceramide in normal and ischemic-reperfused myocardium of rats. Basic Res Cardiol. 2001; 96:267–274.
Article24. Borodzicz S, Czarzasta K, Kuch M, Cudnoch-Jedrzejewska A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015; 14:55.
Article25. Knapp M, Żendzian-Piotrowska M, Błachnio-Zabielska A, Zabielski P, Kurek K, Górski J. Myocardial infarction differentially alters sphingolipid levels in plasma, erythrocytes and platelets of the rat. Basic Res Cardiol. 2012; 107:294.
Article26. Nofer JR, van der Giet M, Tölle M, Wolinska I, von Wnuck Lipinski K, Baba HA, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004; 113:569–581.
Article27. Cavalli AL, Ligutti JA, Gellings NM, Castro E, Page M, Klepper R, et al. The role of TNFα and sphingolipid signaling in cardiac hypoxia: evidence that cardiomyocytes release TNFα and sphingosine. Basic Appl Myol. 2002; 12:167–175.28. Duan HF, Wang H, Yi J, Liu HJ, Zhang QW, Li LB, et al. Adenoviral gene transfer of sphingosine kinase 1 protects heart against ischemia/reperfusion-induced injury and attenuates its postischemic failure. Hum Gene Ther. 2007; 18:1119–1128.
Article29. Caforio AL, Malipiero G, Marcolongo R, Iliceto S. Myocarditis: a clinical overview. Curr Cardiol Rep. 2017; 19:63.
Article30. Rose NR. Viral myocarditis. Curr Opin Rheumatol. 2016; 28:383–389.
Article31. Huber SA. Viral myocarditis and dilated cardiomyopathy: etiology and pathogenesis. Curr Pharm Des. 2016; 22:408–426.
Article32. Martín-Acebes MA, Merino-Ramos T, Blázquez AB, Casas J, Escribano-Romero E, Sobrino F, et al. The composition of West Nile virus lipid envelope unveils a role of sphingolipid metabolism in flavivirus biogenesis. J Virol. 2014; 88:12041–12054.
Article33. Perera R, Riley C, Isaac G, Hopf-Jannasch AS, Moore RJ, Weitz KW, et al. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog. 2012; 8:e1002584.
Article34. Hirata Y, Ikeda K, Sudoh M, Tokunaga Y, Suzuki A, Weng L, et al. Self-enhancement of hepatitis C virus replication by promotion of specific sphingolipid biosynthesis. PLoS Pathog. 2012; 8:e1002860.
Article35. Tan G, Zhou Q, Liu K, Dong X, Li L, Liao W, et al. Cross-platform metabolic profiling deciphering the potential targets of Shenfu injection against acute viral myocarditis in mice. J Pharm Biomed Anal. 2018; 160:1–11.
Article36. Vijayan M, Hahm B. Influenza viral manipulation of sphingolipid metabolism and signaling to modulate host defense system. Scientifica (Cairo). 2014; 2014:793815.
Article37. Huber SA. Autoimmunity in myocarditis: relevance of animal models. Clin Immunol Immunopathol. 1997; 83:93–102.
Article38. Huber SA, Lodge PA. Coxsackievirus B-3 myocarditis in Balb/c mice. Evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984; 116:21–29.39. Lawson CM. Evidence for mimicry by viral antigens in animal models of autoimmune disease including myocarditis. Cell Mol Life Sci. 2000; 57:552–560.
Article40. Kodama M, Okura Y, Aizawa Y, Izumi T. Animal models of autoimmune myocarditis. In : Cooper LT, editor. Myocarditis. Totowa (NJ): Humana Press;2003. p. 197–214.41. Kamiyoshi Y, Takahashi M, Yokoseki O, Yazaki Y, Hirose S, Morimoto H, et al. Mycophenolate mofetil prevents the development of experimental autoimmune myocarditis. J Mol Cell Cardiol. 2005; 39:467–477.
Article42. Velez M, Kohli S, Sabbah HN. Animal models of insulin resistance and heart failure. Heart Fail Rev. 2014; 19:1–13.
Article43. Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008; 52:1793–1799.
Article44. Guha A, Harmancey R, Taegtmeyer H. Nonischemic heart failure in diabetes mellitus. Curr Opin Cardiol. 2008; 23:241–248.
Article45. Alonso N, Moliner P, Mauricio D. Pathogenesis, clinical features and treatment of diabetic cardiomyopathy. Adv Exp Med Biol. 2008; 1067:197–217.
Article46. Russo SB, Ross JS, Cowart LA. Sphingolipids in obesity, type 2 diabetes, and metabolic disease. Handb Exp Pharmacol. 2013; 373–401.
Article47. Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism. 2014; 63:1469–1479.
Article48. Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, et al. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2004; 110:3465–3471.
Article49. Jiang XC, Goldberg IJ, Park TS. Sphingolipids and cardiovascular diseases: lipoprotein metabolism, atherosclerosis and cardiomyopathy. Adv Exp Med Biol. 2011; 721:19–39.
Article50. Lorenzo O, Ramírez E, Picatoste B, Egido J, Tuñón J. Alteration of energy substrates and ROS production in diabetic cardiomyopathy. Mediators Inflamm. 2013; 2013:461967.
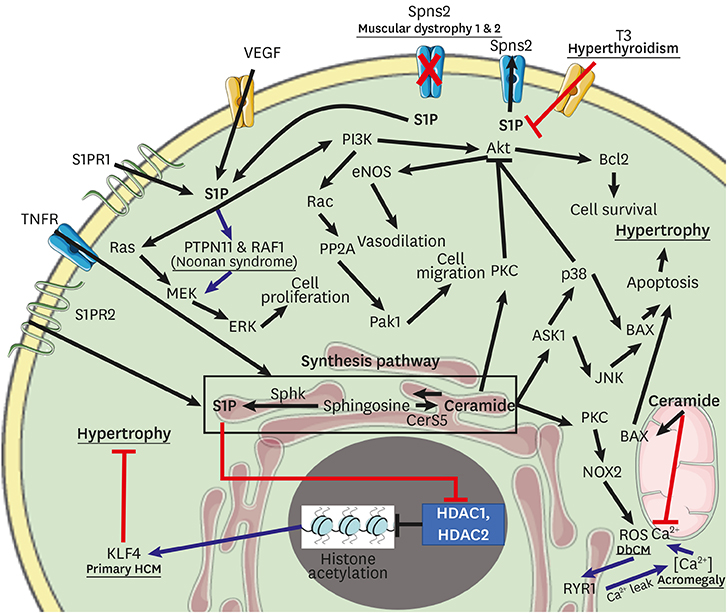
Article51. Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, et al. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest. 2012; 122:3919–3930.
Article52. Hu W, Ross J, Geng T, Brice SE, Cowart LA. Differential regulation of dihydroceramide desaturase by palmitate versus monounsaturated fatty acids: implications for insulin resistance. J Biol Chem. 2011; 286:16596–16605.
Article53. Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011; 121:1402–1411.
Article54. Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech. 2009; 2:454–466.
Article55. Riquelme CA, Magida JA, Harrison BC, Wall CE, Marr TG, Secor SM, et al. Fatty acids identified in the Burmese python promote beneficial cardiac growth. Science. 2011; 334:528–531.
Article56. Chavez JA, Siddique MM, Wang ST, Ching J, Shayman JA, Summers SA. Ceramides and glucosylceramides are independent antagonists of insulin signaling. J Biol Chem. 2014; 289:723–734.
Article57. Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014; 20:687–695.
Article58. Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010; 59:2453–2464.
Article59. de Mello VD, Lankinen M, Schwab U, Kolehmainen M, Lehto S, Seppänen-Laakso T, et al. Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia. 2009; 52:2612–2615.
Article60. Murphy MP. Mitochondrial dysfunction indirectly elevates ROS production by the endoplasmic reticulum. Cell Metab. 2013; 18:145–146.
Article61. Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016; 126:12–22.
Article62. Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol. 2017; 13:79–91.
Article63. Chariyawong P, Rao A, Panikkath D, Panikkath R. Hyperthyroidism-induced dilated cardiomyopathy. Southwest Respir Crit Care Chron. 2019; 7:64–66.
Article64. Klimaite R, Kinderyte M, Dauksaite N, Barsiene L, Zilaitiene B. Pancytopenia and reversible cardiomyopathy-complications of thyrotoxicosis: case report. In : 21st European Congress of Endocrinology; 18-21 May 2019; Lyon, France. Bristol: European Society of Endocrinology;2019.65. Lino CA, Demasi M, Barreto-Chaves ML. Ubiquitin proteasome system (UPS) activation in the cardiac hypertrophy of hyperthyroidism. Mol Cell Endocrinol. 2019; 493:110451.
Article66. Mikłosz A, Łukaszuk B, Chabowski A, Rogowski F, Kurek K, Żendzian-Piotrowska M. Hyperthyroidism evokes myocardial ceramide accumulation. Cell Physiol Biochem. 2015; 35:755–766.
Article67. Pastores GM, Hughes DA. Gaucher disease. GeneReviews® [Internet]. Seattle (WA): University of Washington;2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1269/.68. Riera AR, Schapachnik E, Dubner S. The genetic causes of heart failure a focus on sudden death: from the molecular mechanisms to clinical approach. Lodz: International Society for Holter and Noninvasive Electrocardiology;2006.69. Laks Y, Passwell J. The varied clinical and laboratory manifestations of type II Gaucher's disease. Acta Paediatr Scand. 1987; 76:378–380.
Article70. Casta A, Hayden K, Wolf WJ. Calcification of the ascending aorta and aortic and mitral valves in Gaucher's disease. Am J Cardiol. 1984; 54:1390–1391.
Article71. Bohlega S, Kambouris M, Shahid M, Al Homsi M, Al Sous W. Gaucher disease with oculomotor apraxia and cardiovascular calcification (Gaucher type IIIC). Neurology. 2000; 54:261–263.
Article72. Saraçlar M, Atalay S, Koçak N, Özkutlu S. Gaucher's disease with mitral and aortic involvement: echocardiographic findings. Pediatr Cardiol. 1992; 13:56–58.73. Sharratt GP, Price D, Curtis JA, Cornel G. Gaucher's disease with mitral valve calcification. Pediatr Cardiol. 1992; 13:127–128.
Article74. Karakoyun M, Canda E, Kiran Tasci E, Dogan E, Coker M, Aydogdu S. Two siblings with Gaucher type 3c: different clinical presentations. J Pediatr Endocrinol Metab. 2019; 32:533–536.
Article75. Chabás A, Cormand B, Grinberg D, Burguera JM, Balcells S, Merino JL, et al. Unusual expression of Gaucher's disease: cardiovascular calcifications in three sibs homozygous for the D409H mutation. J Med Genet. 1995; 32:740–742.
Article76. Hein LK, Meikle PJ, Hopwood JJ, Fuller M. Secondary sphingolipid accumulation in a macrophage model of Gaucher disease. Mol Genet Metab. 2007; 92:336–345.
Article77. Goldman ME, Cantor R, Schwartz MF, Baker M, Desnick RJ. Echocardiographic abnormalities and disease severity in Fabry's disease. J Am Coll Cardiol. 1986; 7:1157–1161.
Article78. Sachdev B, Takenaka T, Teraguchi H, Tei C, Lee P, McKenna WJ, et al. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation. 2002; 105:1407–1411.
Article79. Chimenti C, Pieroni M, Morgante E, Antuzzi D, Russo A, Russo MA, et al. Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation. 2004; 110:1047–1053.
Article80. Conway R. The sphingolipidoses. In : Rubin IL, Merrick J, Greydanus DE, Patel DR, editors. Health care for people with intellectual and developmental disabilities across the lifespan. Zurich: Springer International;2016. p. 659–682.81. Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, et al. An atypical variant of Fabry's disease in men with left ventricular hypertrophy. N Engl J Med. 1995; 333:288–293.
Article82. Elleder M, Bradová V, Smíd F, Budĕsínský M, Harzer K, Kustermann-Kuhn B, et al. Cardiocyte storage and hypertrophy as a sole manifestation of Fabry's disease. Report on a case simulating hypertrophic non-obstructive cardiomyopathy. Virchows Arch A Pathol Anat Histopathol. 1990; 417:449–455.
Article83. Pieruzzi F, Pieroni M, Zachara E, Marziliano N, Morrone A, Cecchi F. Heart involvement in Anderson-Fabry disease: Italian recommendations for diagnostic, follow-up and therapeutic management. G Ital Cardiol (Rome). 2015; 16:630–638.84. Kampmann C, Linhart A, Baehner F, Palecek T, Wiethoff CM, Miebach E, et al. Onset and progression of the Anderson-Fabry disease related cardiomyopathy. Int J Cardiol. 2008; 130:367–373.
Article85. Pieroni M, Chimenti C, De Cobelli F, Morgante E, Del Maschio A, Gaudio C, et al. Fabry's disease cardiomyopathy: echocardiographic detection of endomyocardial glycosphingolipid compartmentalization. J Am Coll Cardiol. 2006; 47:1663–1671.86. Kounas S, Demetrescu C, Pantazis AA, Keren A, Lee PJ, Hughes D, et al. The binary endocardial appearance is a poor discriminator of Anderson-Fabry disease from familial hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008; 51:2058–2061.
Article87. Yogasundaram H, Nikhanj A, Putko BN, Boutin M, Jain-Ghai S, Khan A, et al. Elevated inflammatory plasma biomarkers in patients with fabry disease: a critical link to heart failure with preserved ejection fraction. J Am Heart Assoc. 2018; 7:e009098.
Article88. Ommen SR, Nishimura RA, Edwards WD. Fabry disease: a mimic for obstructive hypertrophic cardiomyopathy? Heart. 2003; 89:929–930.
Article89. Seward JB, Casaclang-Verzosa G. Infiltrative cardiovascular diseases: cardiomyopathies that look alike. J Am Coll Cardiol. 2010; 55:1769–1779.90. Vitner EB, Platt FM, Futerman AH. Common and uncommon pathogenic cascades in lysosomal storage diseases. J Biol Chem. 2010; 285:20423–20427.
Article91. Brunetti-Pierri N, Scaglia F. GM1 gangliosidosis: review of clinical, molecular, and therapeutic aspects. Mol Genet Metab. 2008; 94:391–396.
Article92. Lin HC, Tsai FJ, Shen WC, Tsai CH, Peng CT. Infantile form GM1 gangliosidosis with dilated cardiomyopathy: a case report. Acta Paediatr. 2000; 89:880–883.
Article93. Rosenberg H, Frewen TC, Li MD, Gordon BL, Jung JH, Finlay JP, et al. Cardiac involvement in diseases characterized by β-galactosidase deficiency. J Pediatr. 1985; 106:78–80.
Article94. Hadley RN, Hagstrom JW. Cardiac lesions in a patient with familial neurovisceral lipidosis (generalized gangliosidosis). Am J Clin Pathol. 1971; 55:237–240.
Article95. McMinn TR Jr, Ross J Jr. Hereditary dilated cardiomyopathy. Clin Cardiol. 1995; 18:7–15.
Article96. Benson PF, Barbarik A, Brown SP, Mann TP. GM1-generalized gangliosidosis variant with cardiomegaly. Postgrad Med J. 1976; 52:159–165.
Article97. Rodriguez-Torres R, Schneck L, Kleinberg W. Electrocardiographic and biochemical abnormalities in Tay-Sachs disease. Bull N Y Acad Med. 1971; 47:717–730.
Article98. Gilbert-Barness E. Metabolic cardiomyopathy and conduction system defects in children. Ann Clin Lab Sci. 2004; 34:15–34.99. Brown M, Goldstein J, Fredrickson D. Familial type 3 hyperlipoproteinemia (dysbetalipoproteinemia). In : Stanbury JB, Wyngaarden JB, Fredrickson DS, editors. Metabolic basis of inherited disease. New York (NY): McGraw-Hill;1983.100. Blieden LC, Desnick RJ, Carter JB, Krivit W, Moller JH, Sharp HL. Cardiac involvement in Sandhoff's disease. Inborn error of glycosphingolipid metabolism. Am J Cardiol. 1974; 34:83–88.101. Kohlschütter A, Hausdorf G. Primary (genetic) cardiomyopathies in infancy. A survey of possible disorders and guidelines for diagnosis. Eur J Pediatr. 1986; 145:454–459.
Article102. Verhaert D, Richards K, Rafael-Fortney JA, Raman SV. Cardiac involvement in patients with muscular dystrophies: magnetic resonance imaging phenotype and genotypic considerations. Circ Cardiovasc Imaging. 2011; 4:67–76.103. Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci U S A. 1995; 92:8655–8659.
Article104. Li S, Song KS, Lisanti MP. Expression and characterization of recombinant caveolin. Purification by polyhistidine tagging and cholesterol-dependent incorporation into defined lipid membranes. J Biol Chem. 1996; 271:568–573.105. Gardin JM, Lauer MS. Left ventricular hypertrophy: the next treatable, silent killer? JAMA. 2004; 292:2396–2398.106. Spijkers LJ, Janssen BJ, Nelissen J, Meens MJ, Wijesinghe D, Chalfant CE, et al. Antihypertensive treatment differentially affects vascular sphingolipid biology in spontaneously hypertensive rats. PLoS One. 2011; 6:e29222.
Article107. Spijkers LJ, van den Akker RF, Janssen BJ, Debets JJ, De Mey JG, Stroes ES, et al. Hypertension is associated with marked alterations in sphingolipid biology: a potential role for ceramide. PLoS One. 2011; 6:e21817.
Article108. Kuroda K, Kato TS, Amano A. Hypertensive cardiomyopathy: a clinical approach and literature review. World J Hypertens. 2015; 5:41–52.
Article109. Fenger M, Linneberg A, Jørgensen T, Madsbad S, Søbye K, Eugen-Olsen J, et al. Genetics of the ceramide/sphingosine-1-phosphate rheostat in blood pressure regulation and hypertension. BMC Genet. 2011; 12:44.
Article110. Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, et al. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation. 2002; 106:2250–2256.111. Hemmings DG. Signal transduction underlying the vascular effects of sphingosine 1-phosphate and sphingosylphosphorylcholine. Naunyn Schmiedebergs Arch Pharmacol. 2006; 373:18–29.
Article112. Ellis ER, Josephson ME. Heart failure and tachycardia-induced cardiomyopathy. Curr Heart Fail Rep. 2013; 10:296–306.
Article113. Ellis ER, Josephson ME. What about tachycardia-induced cardiomyopathy? Arrhythm Electrophysiol Rev. 2013; 2:82–90.
Article114. Gopinathannair R, Etheridge SP, Marchlinski FE, Spinale FG, Lakkireddy D, Olshansky B. Arrhythmia-induced cardiomyopathies: mechanisms, recognition, and management. J Am Coll Cardiol. 2015; 66:1714–1728.115. Chugh SS, Shen WK, Luria DM, Smith HC. First evidence of premature ventricular complex-induced cardiomyopathy: a potentially reversible cause of heart failure. J Cardiovasc Electrophysiol. 2000; 11:328–329.
Article116. Grogan M, Smith HC, Gersh BJ, Wood DL. Left ventricular dysfunction due to atrial fibrillation in patients initially believed to have idiopathic dilated cardiomyopathy. Am J Cardiol. 1992; 69:1570–1573.
Article117. Luchsinger JA, Steinberg JS. Resolution of cardiomyopathy after ablation of atrial flutter. J Am Coll Cardiol. 1998; 32:205–210.
Article118. Cruz FE, Cheriex EC, Smeets JL, Atié J, Peres AK, Penn OC, et al. Reversibility of tachycardia-induced cardiomyopathy after cure of incessant supraventricular tachycardia. J Am Coll Cardiol. 1990; 16:739–744.
Article119. Jaggarao NS, Nanda AS, Daubert JP. Ventricular tachycardia induced cardiomyopathy: improvement with radiofrequency ablation. Pacing Clin Electrophysiol. 1996; 19:505–508.
Article120. Wojcik B, Baranowski M, Chabowski A, Gorski J. Effect of atrial pacing on the level of bioactive sphingolipids in the heart ventricles of the rat. J Physiol Pharmacol. 2015; 66:385–389.121. Wojcik B, Miklosz A, Zabielski P, Chabowski A, Gorski J. Effect of tachycardia on mRNA and protein expression of the principal components of the lipolytic system in the rat's heart ventricles. J Physiol Pharmacol. 2017; 68:731–736.122. Hedley PL, Jørgensen P, Schlamowitz S, Wangari R, Moolman-Smook J, Brink PA, et al. The genetic basis of long QT and short QT syndromes: a mutation update. Hum Mutat. 2009; 30:1486–1511.
Article123. Bai Y, Wang J, Shan H, Lu Y, Zhang Y, Luo X, et al. Sphingolipid metabolite ceramide causes metabolic perturbation contributing to HERG K+ channel dysfunction. Cell Physiol Biochem. 2007; 20:429–440.
Article124. Marbán E. Cardiac channelopathies. Nature. 2002; 415:213–218.
Article125. Itoh G, Tamura J, Suzuki M, Suzuki Y, Ikeda H, Koike M, et al. DNA fragmentation of human infarcted myocardial cells demonstrated by the nick end labeling method and DNA agarose gel electrophoresis. Am J Pathol. 1995; 146:1325–1331.126. Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL, et al. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996; 98:2854–2865.
Article127. Laderoute KR, Webster KA. Hypoxia/reoxygenation stimulates Jun kinase activity through redox signaling in cardiac myocytes. Circ Res. 1997; 80:336–344.
Article128. Mallat Z, Tedgui A, Fontaliran F, Frank R, Durigon M, Fontaine G. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N Engl J Med. 1996; 335:1190–1196.
Article129. Tanaka M, Ito H, Adachi S, Akimoto H, Nishikawa T, Kasajima T, et al. Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circ Res. 1994; 75:426–433.
Article130. Hiroe M, Toyozaki T. Pathogenesis of myocardial injury and cell death in myocarditis: its relation to the fas/fas ligand pathway. In : Kitabatake A, Sasayama S, Francis GS, Okamoto H, editors. Heart failure. Tokyo: Springer;2000. p. 57–69.131. Robert P, Tsui P, Laville MP, Livi GP, Sarau HM, Bril A, et al. EDG1 receptor stimulation leads to cardiac hypertrophy in rat neonatal myocytes. J Mol Cell Cardiol. 2001; 33:1589–1606.
Article132. Landeen LK, Aroonsakool N, Haga JH, Hu BS, Giles WR. Sphingosine-1-phosphate receptor expression in cardiac fibroblasts is modulated by in vitro culture conditions. Am J Physiol Heart Circ Physiol. 2007; 292:H2698–H2711.133. Peters SL, Alewijnse AE. Sphingosine-1-phosphate signaling in the cardiovascular system. Curr Opin Pharmacol. 2007; 7:186–192.
Article134. Kurdi M, Booz GW. Three 4-letter words of hypertension-related cardiac hypertrophy: TRPC, mTOR, and HDAC. J Mol Cell Cardiol. 2011; 50:964–971.
Article135. Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009; 325:1254–1257.
Article136. Yan H, Yi S, Zhuang H, Wu L, Wang DW, Jiang J. Sphingosine-1-phosphate ameliorates the cardiac hypertrophic response through inhibiting the activity of histone deacetylase-2. Int J Mol Med. 2018; 41:1704–1714.
Article137. Alonso-Montes C, Naves-Diaz M, Fernandez-Martin JL, Rodriguez-Reguero J, Moris C, Coto E, et al. New polymorphisms in human MEF2C gene as potential modifier of hypertrophic cardiomyopathy. Mol Biol Rep. 2012; 39:8777–8785.
Article138. Kolesnick RN, Haimovitz-Friedman A, Fuks Z. The sphingomyelin signal transduction pathway mediates apoptosis for tumor necrosis factor, Fas, and ionizing radiation. Biochem Cell Biol. 1994; 72:471–474.
Article139. Papathanasiou S, Rickelt S, Soriano M, Schips T, Maier HJ, Davos CH, et al. A novel mechanism of cardioprotection through TNF-α-induced ectopic expression of keratins K8 and K18. Nat Med. 2015; 21:1076.
Article140. Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, et al. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC ε knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002; 282:H1970–H1977.141. Bielawska AE, Shapiro JP, Jiang L, Melkonyan HS, Piot C, Wolfe CL, et al. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am J Pathol. 1997; 151:1257–1263.142. Tao R, Hoover HE, Honbo N, Kalinowski M, Alano CC, Karliner JS, et al. High-density lipoprotein determines adult mouse cardiomyocyte fate after hypoxia-reoxygenation through lipoprotein-associated sphingosine 1-phosphate. Am J Physiol Heart Circ Physiol. 2010; 298:H1022–H1028.
Article143. Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007; 293:H3150–H3158.
Article144. Solaro RJ, Sheehan KA, Lei M, Ke Y. The curious role of sarcomeric proteins in control of diverse processes in cardiac myocytes. J Gen Physiol. 2010; 136:13–19.
Article145. Brizuela L, Rábano M, Peña A, Gangoiti P, Macarulla JM, Trueba M, et al. Sphingosine 1-phosphate: a novel stimulator of aldosterone secretion. J Lipid Res. 2006; 47:1238–1249.
Article146. Marino A, Sakamoto T, Robador PA, Tomita K, Levi R. S1P receptor 1-mediated anti–renin-angiotensin system cardioprotection: pivotal role of mast cell aldehyde dehydrogenase type 2. J Pharmacol Exp Ther. 2017; 362:230–242.
Article147. Haass NK, Nassif N, McGowan EM. Switching the sphingolipid rheostat in the treatment of diabetes and cancer comorbidity from a problem to an advantage. BioMed Res Int. 2015; 2015:165105.
Article148. Yin Z, Fan L, Wei L, Gao H, Zhang R, Tao L, et al. FTY720 protects cardiac microvessels of diabetes: a critical role of S1P1/3 in diabetic heart disease. PLoS One. 2012; 7:e42900.
Article149. Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci U S A. 2003; 100:10664–10669.
Article150. Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012; 485:333–338.
Article151. Alewijnse AE, Peters SL, Michel MC. Cardiovascular effects of sphingosine-1-phosphate and other sphingomyelin metabolites. Br J Pharmacol. 2004; 143:666–684.
Article152. de Faria Poloni J, Chapola H, Feltes BC, Bonatto D. The importance of sphingolipids and reactive oxygen species in cardiovascular development. Biol Cell. 2014; 106:167–181.
Article153. Hussein AA, El-Dken ZH, Barakat N, Abol-Enein H. Renal ischaemia/reperfusion injury: possible role of aquaporins. Acta Physiol (Oxf). 2012; 204:308–316.
Article154. Xu L, Meissner G. Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+. Biophys J. 1998; 75:2302–2312.
Article155. Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008; 70:23–49.
Article156. Fearnley CJ, Roderick HL, Bootman MD. Calcium signaling in cardiac myocytes. Cold Spring Harb Perspect Biol. 2011; 3:a004242.
Article157. Paur HE. Adrenaline-mediated biased agonism at the B2 adrenoceptor in an in vivo model of takutsubo cardiomyopathy. London: Imperial College London;2012.158. Kitatani K, Idkowiak-Baldys J, Bielawski J, Taha TA, Jenkins RW, Senkal CE, et al. Protein kinase C-induced activation of a ceramide/protein phosphatase 1 pathway leading to dephosphorylation of p38 MAPK. J Biol Chem. 2006; 281:36793–36802.
Article159. Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008; 20:1010–1018.
Article160. Berry C, Touyz R, Dominiczak AF, Webb RC, Johns DG. Angiotensin receptors: signaling, vascular pathophysiology, and interactions with ceramide. Am J Physiol Heart Circ Physiol. 2001; 281:H2337–H2365.161. Gallinat S, Busche S, Schütze S, Krönke M, Unger T. AT2 receptor stimulation induces generation of ceramides in PC12W cells. FEBS Lett. 1999; 443:75–79.
Article162. Johns DG, Osborn H, Webb RC. Ceramide: a novel cell signaling mechanism for vasodilation. Biochem Biophys Res Commun. 1997; 237:95–97.
Article163. Jones MJ, Murray AW. Evidence that ceramide selectively inhibits protein kinase C-α translocation and modulates bradykinin activation of phospholipase D. J Biol Chem. 1995; 270:5007–5013.
Article164. Lee JY, Hannun YA, Obeid LM. Ceramide inactivates cellular protein kinase Cα. J Biol Chem. 1996; 271:13169–13174.
Article165. Zhang DX, Zou AP, Li PL. Ceramide reduces endothelium-dependent vasodilation by increasing superoxide production in small bovine coronary arteries. Circ Res. 2001; 88:824–831.
Article166. Kennedy S, Kane KA, Pyne NJ, Pyne S. Targeting sphingosine-1-phosphate signalling for cardioprotection. Curr Opin Pharmacol. 2009; 9:194–201.
Article167. Sun M, Miao Y, Wang P, Miao L, Liu L, Liu J. Urinary metabonomics study of heart failure patients with HILIC and RPLC separation coupled to TOF–MS. Chromatographia. 2014; 77:249–255.
Article168. Glaros EN, Kim WS, Wu BJ, Suarna C, Quinn CM, Rye KA, et al. Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem Pharmacol. 2007; 73:1340–1346.
Article169. Liu W, Min Z, Naumann R, Ke Y, Ulm S, Jin J, et al. PAK1 is a novel signal transducer attenuating cardiac hypertrophy. Circulation. 2011; 124:2702–2715.170. Wang Y, Tsui H, Ke Y, Shi Y, Li Y, Davies L, et al. Pak1 is required to maintain ventricular Ca2+ homeostasis and electrophysiological stability through SERCA2a regulation in mice. Circ Arrhythm Electrophysiol. 2014; 7:938–948.
Article171. Liu W, Zi M, Tsui H, Chowdhury SK, Zeef L, Meng QJ, et al. A novel immunomodulator, FTY-720 reverses existing cardiac hypertrophy and fibrosis from pressure overload by targeting NFAT (nuclear factor of activated T-cells) signaling and periostin. Circ Heart Fail. 2013; 6:833–844.
Article172. Chun J, Brinkmann V. A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya). Discov Med. 2011; 12:213–228.173. He L, Kim T, Long Q, Liu J, Wang P, Zhou Y, et al. Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation. 2012; 126:1705–1716.
Article174. Drake JI, Gomez-Arroyo J, Dumur CI, Kraskauskas D, Natarajan R, Bogaard HJ, et al. Chronic carvedilol treatment partially reverses the right ventricular failure transcriptional profile in experimental pulmonary hypertension. Physiol Genomics. 2013; 45:449–461.
Article175. Marcus GM, Glidden DV, Polonsky B, Zareba W, Smith LM, Cannom DS, et al. Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: a report from the North American ARVC Registry. J Am Coll Cardiol. 2009; 54:609–615.
Article176. Wen-Ting S, Fa-Feng C, Li X, Cheng-Ren L, Jian-Xun L. Chinese medicine shenfu injection for heart failure: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2012; 2012:713149.
Article177. Ogawa R, Takahashi M, Hirose S, Morimoto H, Ise H, Murakami T, et al. A novel sphingosine-1-phosphate receptor agonist KRP-203 attenuates rat autoimmune myocarditis. Biochem Biophys Res Commun. 2007; 361:621–628.
Article178. Kitabayashi H, Isobe M, Watanabe N, Suzuki J, Yazaki Y, Sekiguchi M. FTY720 prevents development of experimental autoimmune myocarditis through reduction of circulating lymphocytes. J Cardiovasc Pharmacol. 2000; 35:410–416.
Article179. Sugita K, Kabashima K, Sakabe J, Yoshiki R, Tanizaki H, Tokura Y. FTY720 regulates bone marrow egress of eosinophils and modulates late-phase skin reaction in mice. Am J Pathol. 2010; 177:1881–1887.
Article180. Sawicka E, Zuany-Amorim C, Manlius C, Trifilieff A, Brinkmann V, Kemeny DM, et al. Inhibition of Th1- and Th2-mediated airway inflammation by the sphingosine 1-phosphate receptor agonist FTY720. J Immunol. 2003; 171:6206–6214.
Article181. Nixon GF. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol. 2009; 158:982–993.
Article182. Bennett LL, Turcotte K. Eliglustat tartrate for the treatment of adults with type 1 Gaucher disease. Drug Des Devel Ther. 2015; 9:4639–4647.
Article183. Shayman JA. The design and clinical development of inhibitors of glycosphingolipid synthesis: will invention be the mother of necessity? Trans Am Clin Climatol Assoc. 2013; 124:46–60.184. Shayman JA. Developing novel chemical entities for the treatment of lysosomal storage disorders: an academic perspective. Am J Physiol Renal Physiol. 2015; 309:F996–F999.
Article185. Shayman JA, Larsen SD. The development and use of small molecule inhibitors of glycosphingolipid metabolism for lysosomal storage diseases. J Lipid Res. 2014; 55:1215–1225.
Article186. Colussi DJ, Jacobson MA. Patient-derived phenotypic high-throughput assay to identify small molecules restoring lysosomal function in Tay-Sachs disease. SLAS Discov. 2019; 24:295–303.
Article187. Yue WW, Mackinnon S, Bezerra GA. Substrate reduction therapy for inborn errors of metabolism. Emerg Top Life Sci. 2019; 3:63–73.
Article188. Brady RO. Enzyme replacement for lysosomal diseases. Annu Rev Med. 2006; 57:283–296.
Article189. Futerman AH, Sussman JL, Horowitz M, Silman I, Zimran A. New directions in the treatment of Gaucher disease. Trends Pharmacol Sci. 2004; 25:147–151.
Article190. Cox TM, Cachón-González MB. The cellular pathology of lysosomal diseases. J Pathol. 2012; 226:241–254.
Article191. Schiffmann R, Kopp JB, Austin HA 3rd, Sabnis S, Moore DF, Weibel T, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001; 285:2743–2749.192. Zarate YA, Hopkin RJ. Fabry's disease. Lancet. 2008; 372:1427–1435.
Article193. Linhart A, Elliott PM. The heart in Anderson-Fabry disease and other lysosomal storage disorders. Heart. 2007; 93:528–535.
Article194. Frustaci A, Chimenti C, Ricci R, Natale L, Russo MA, Pieroni M, et al. Improvement in cardiac function in the cardiac variant of Fabry's disease with galactose-infusion therapy. N Engl J Med. 2001; 345:25–32.
Article195. Young E, Mills K, Morris P, Vellodi A, Lee P, Waldek S, et al. Is globotriaosylceramide a useful biomarker in Fabry disease? Acta Paediatr Suppl. 2005; 94:51–54.
Article196. Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, et al. Safety and efficacy of recombinant human α-galactosidase A replacement therapy in Fabry's disease. N Engl J Med. 2001; 345:9–16.
Article197. Nicholls K, Olivotto I, Ohashi T, Williams H, Jain V, Skuban N. The effects of long-term migalastat treatment in Fabry disease patients previously treated with enzyme replacement therapy who have migalastat-amenable variants with low alpha-galactosidase A response in the in vitro migalastat amenability assay. Mol Genet Metab. 2019; 126:S108.
Article198. Narita A, Shirai K, Itamura S, Matsuda A, Ishihara A, Matsushita K, et al. Ambroxol chaperone therapy for neuronopathic Gaucher disease: a pilot study. Ann Clin Transl Neurol. 2016; 3:200–215.
Article199. Maegawa GH, Tropak MB, Buttner JD, Rigat BA, Fuller M, Pandit D, et al. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J Biol Chem. 2009; 284:23502–23516.
Article200. Clarke JT, Mahuran DJ, Sathe S, Kolodny EH, Rigat BA, Raiman JA, et al. An open-label Phase I/II clinical trial of pyrimethamine for the treatment of patients affected with chronic GM2 gangliosidosis (Tay-Sachs or Sandhoff variants). Mol Genet Metab. 2011; 102:6–12.
Article201. Lieberman AP, Puertollano R, Raben N, Slaugenhaupt S, Walkley SU, Ballabio A. Autophagy in lysosomal storage disorders. Autophagy. 2012; 8:719–730.
Article202. Seranova E, Connolly KJ, Zatyka M, Rosenstock TR, Barrett T, Tuxworth RI, et al. Dysregulation of autophagy as a common mechanism in lysosomal storage diseases. Essays Biochem. 2017; 61:733–749.
Article203. Villamizar-Schiller IT, Pabón LA, Hufnagel SB, Serrano NC, Karl G, Jefferies JL, et al. Neurological and cardiac responses after treatment with miglustat and a ketogenic diet in a patient with Sandhoff disease. Eur J Med Genet. 2015; 58:180–183.
Article204. Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007; 5:167–179.
Article205. Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012; 15:585–594.
Article206. Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, et al. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem. 2005; 280:10284–10289.
Article207. Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008; 29:381–402.
Article208. Goodwin AJ, Leadley SR, O'neill L, Duffield PJ, McKechnie MT, Pugh S. Process and apparatus for plasma coating, substrates coated by this metod or apparatus. Patent No. WO 2005110626A2. 2008.209. Kotronen A, Seppänen-Laakso T, Westerbacka J, Kiviluoto T, Arola J, Ruskeepää AL, et al. Comparison of lipid and fatty acid composition of the liver, subcutaneous and intra-abdominal adipose tissue, and serum. Obesity (Silver Spring). 2010; 18:937–944.
Article210. Kolter T. A view on sphingolipids and disease. Chem Phys Lipids. 2011; 164:590–606.
Article211. Egom EE, Mamas MA, Chacko S, Stringer SE, Charlton-Menys V, El-Omar M, et al. Serum sphingolipids level as a novel potential marker for early detection of human myocardial ischaemic injury. Front Physiol. 2013; 4:130.
Article212. Schatz P, Witt H, Peter E, Ternes P, Mappes P, Katus HA, et al. Means and methods for diagnosing heart failure on the basis of cholesterol parameters, sphingomyelins and/or triacylglycerols. Patent No. WO2016016258A1. 2017.213. Fine B, Marx A, Topkara V, Gomez E, Vunjak-Novakovic G, Colombo P. An integrated analysis of metabolomics after left ventricular assist device implantation. J Heart Lung Transplant. 2017; 36:S93.
Article214. Topilsky Y, Pereira NL, Shah DK, Boilson B, Schirger JA, Kushwaha SS, et al. Left ventricular assist device therapy in patients with restrictive and hypertrophic cardiomyopathy. Circ Heart Fail. 2011; 4:266–275.
Article215. Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNF α and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol. 2002; 34:509–518.
Article216. Vessey DA, Li L, Honbo N, Karliner JS. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am J Physiol Heart Circ Physiol. 2009; 297:H1429–H1435.
Article217. Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, Young TR, et al. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003; 146:62–68.
Article218. Klevstig M, Ståhlman M, Lundqvist A, Scharin Täng M, Fogelstrand P, Adiels M, et al. Targeting acid sphingomyelinase reduces cardiac ceramide accumulation in the post-ischemic heart. J Mol Cell Cardiol. 2016; 93:69–72.
Article219. Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012; 51:50–62.
Article220. Jiang Y, DiVittore NA, Kaiser JM, Shanmugavelandy SS, Fritz JL, Heakal Y, et al. Combinatorial therapies improve the therapeutic efficacy of nanoliposomal ceramide for pancreatic cancer. Cancer Biol Ther. 2011; 12:574–585.
Article221. Kester M, Bassler J, Fox TE, Carter CJ, Davidson JA, Parette MR. Preclinical development of a C6-ceramide NanoLiposome, a novel sphingolipid therapeutic. Biol Chem. 2015; 396:737–747.
Article222. Hankins JL, Doshi UA, Haakenson JK, Young MM, Barth BM, Kester M. The therapeutic potential of nanoscale sphingolipid technologies. Handb Exp Pharmacol. 2013; 197–210.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Myocardial Infarction after a Bee Sting
- In Silico Analysis for Sphingolipid Metabolism-Related Genes in Human Kidney Clear Cell Carcinoma Using The Cancer Genome Atlas.
- Altered Sphingolipid Metabolism Is Associated With Asthma Phenotype in House Dust Mite-Allergic Patients
- A Case of Kounis Syndrome Induced by a Non-Steroidal Anti-Inflammatory Drug
- Novel inflammatory biomarkers in acute coronary syndrome