J Lipid Atheroscler.
2020 Jan;9(1):8-22. 10.12997/jla.2020.9.1.8.
mTOR-coordinated Post-Transcriptional Gene Regulations: from Fundamental to Pathogenic Insights
- Affiliations
-
- 1Department of Biochemistry, Molecular Biology and Biophysics, College of Biological Sciences, University of Minnesota, Minneapolis, MN, USA. jyong@umn.edu
- KMID: 2470798
- DOI: http://doi.org/10.12997/jla.2020.9.1.8
Abstract
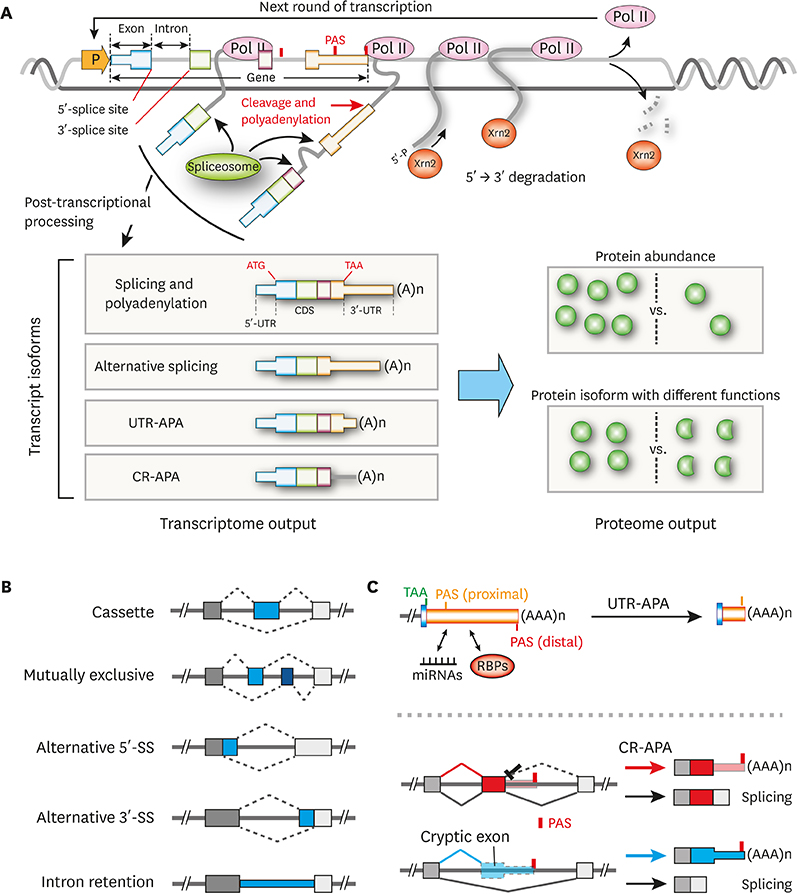
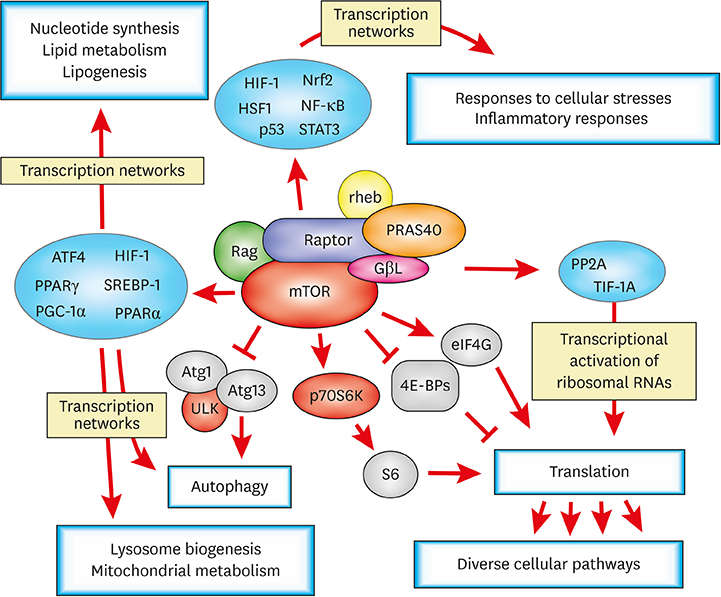
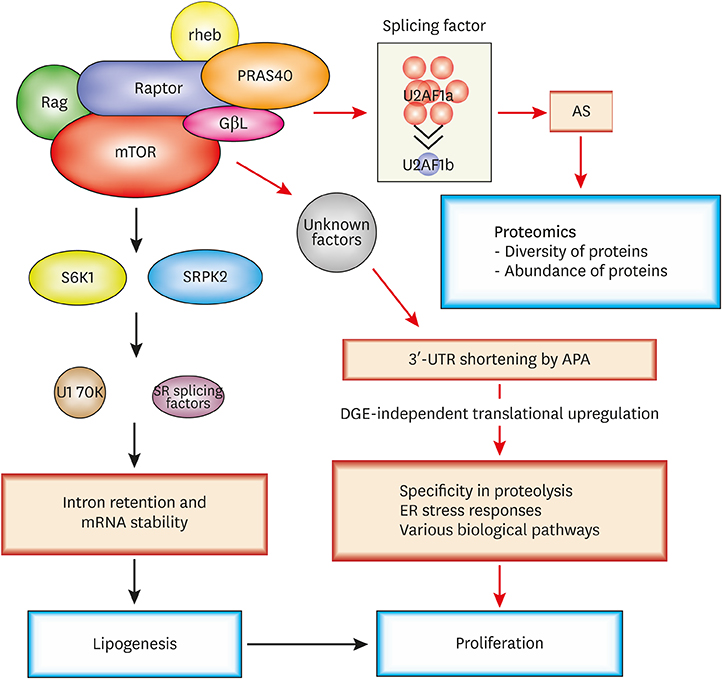
- Post-transcriptional regulations of mRNA transcripts such as alternative splicing and alternative polyadenylation can affect the expression of genes without changing the transcript levels. Recent studies have demonstrated that these post-transcriptional events can have significant physiological impacts on various biological systems and play important roles in the pathogenesis of a number of diseases, including cancers. Nevertheless, how cellular signaling pathways control these post-transcriptional processes in cells are not very well explored in the field yet. The mammalian target of rapamycin complex 1 (mTORC1) pathway plays a key role in sensing cellular nutrient and energy status and regulating the proliferation and growth of cells by controlling various anabolic and catabolic processes. Dysregulation of mTORC1 pathway can tip the metabolic balance of cells and is associated with a number of pathological conditions, including various types of cancers, diabetes, and cardiovascular diseases. Numerous reports have shown that mTORC1 controls its downstream pathways through translational and/or transcriptional regulation of the expression of key downstream effectors. And, recent studies have also shown that mTORC1 can control downstream pathways via post-transcriptional regulations. In this review, we will discuss the roles of post-transcriptional processes in gene expression regulations and how mTORC1-mediated post-transcriptional regulations contribute to cellular physiological changes. We highlight post-transcriptional regulation as an additional layer of gene expression control by mTORC1 to steer cellular biology. These emphasize the importance of studying post-transcriptional events in transcriptome datasets for gaining a fuller understanding of gene expression regulations in the biological systems of interest.
Keyword
MeSH Terms
Figure
Reference
-
1. El Marabti E, Younis I. The cancer spliceome: reprograming of alternative splicing in cancer. Front Mol Biosci. 2018; 5:80.
Article2. David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes Dev. 2010; 24:2343–2364.
Article3. Tian B, Manley JL. Alternative cleavage and polyadenylation: the long and short of it. Trends Biochem Sci. 2013; 38:312–320.
Article4. Di Giammartino DC, Nishida K, Manley JL. Mechanisms and consequences of alternative polyadenylation. Mol Cell. 2011; 43:853–866.
Article5. Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009; 122:3589–3594.
Article6. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012; 149:274–293.
Article7. Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009; 10:307–318.
Article8. Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013; 126:1713–1719.
Article9. Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012; 485:55–61.
Article10. Herzel L, Ottoz DS, Alpert T, Neugebauer KM. Splicing and transcription touch base: co-transcriptional spliceosome assembly and function. Nat Rev Mol Cell Biol. 2017; 18:637–650.
Article11. Hicks MJ, Yang CR, Kotlajich MV, Hertel KJ. Linking splicing to Pol II transcription stabilizes pre-mRNAs and influences splicing patterns. PLoS Biol. 2006; 4:e147.
Article12. David CJ, Boyne AR, Millhouse SR, Manley JL. The RNA polymerase II C-terminal domain promotes splicing activation through recruitment of a U2AF65-Prp19 complex. Genes Dev. 2011; 25:972–983.
Article13. Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008; 40:1413–1415.
Article14. Huelga SC, Vu AQ, Arnold JD, Liang TY, Liu PP, Yan BY, et al. Integrative genome-wide analysis reveals cooperative regulation of alternative splicing by hnRNP proteins. Cell Reports. 2012; 1:167–178.
Article15. Zhou Z, Fu XD. Regulation of splicing by SR proteins and SR protein-specific kinases. Chromosoma. 2013; 122:191–207.
Article16. Shen S, Park JW, Lu ZX, Lin L, Henry MD, Wu YN, et al. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci U S A. 2014; 111:E5593–E5601.
Article17. Wang Y, Liu J, Huang BO, Xu YM, Li J, Huang LF, et al. Mechanism of alternative splicing and its regulation. Biomed Rep. 2015; 3:152–158.
Article18. Rosenstiel P, Huse K, Franke A, Hampe J, Reichwald K, Platzer C, et al. Functional characterization of two novel 5′ untranslated exons reveals a complex regulation of NOD2 protein expression. BMC Genomics. 2007; 8:472.
Article19. Wang G, Guo X, Floros J. Differences in the translation efficiency and mRNA stability mediated by 5′-UTR splice variants of human SP-A1 and SP-A2 genes. Am J Physiol Lung Cell Mol Physiol. 2005; 289:L497–L508.
Article20. Yeh HS, Yong J. Alternative polyadenylation of mRNAs: 3′-untranslated region matters in gene expression. Mol Cells. 2016; 39:281–285.
Article21. Zhang X, Virtanen A, Kleiman FE. To polyadenylate or to deadenylate: that is the question. Cell Cycle. 2010; 9:4437–4449.22. Proudfoot NJ. Ending the message: poly(A) signals then and now. Genes Dev. 2011; 25:1770–1782.
Article23. Kornblihtt AR. Shortcuts to the end. Nat Struct Mol Biol. 2004; 11:1156–1157.
Article24. West S, Gromak N, Proudfoot NJ. Human 5′ → 3′ exonuclease Xrn2 promotes transcription termination at co-transcriptional cleavage sites. Nature. 2004; 432:522–525.
Article25. Kim M, Krogan NJ, Vasiljeva L, Rando OJ, Nedea E, Greenblatt JF, et al. The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature. 2004; 432:517–522.
Article26. Elkon R, Ugalde AP, Agami R. Alternative cleavage and polyadenylation: extent, regulation and function. Nat Rev Genet. 2013; 14:496–506.
Article27. Martin G, Gruber AR, Keller W, Zavolan M. Genome-wide analysis of pre-mRNA 3′ end processing reveals a decisive role of human cleavage factor I in the regulation of 3′ UTR length. Cell Reports. 2012; 1:753–763.
Article28. Masamha CP, Xia Z, Yang J, Albrecht TR, Li M, Shyu AB, et al. CFIm25 links alternative polyadenylation to glioblastoma tumour suppression. Nature. 2014; 510:412–416.
Article29. Yang Q, Gilmartin GM, Doublié S. Structural basis of UGUA recognition by the Nudix protein CFIm25 and implications for a regulatory role in mRNA 3′ processing. Proc Natl Acad Sci U S A. 2010; 107:10062–10067.
Article30. Takagaki Y, Seipelt RL, Peterson ML, Manley JL. The polyadenylation factor CstF-64 regulates alternative processing of IgM heavy chain pre-mRNA during B cell differentiation. Cell. 1996; 87:941–952.
Article31. Bava FA, Eliscovich C, Ferreira PG, Miñana B, Ben-Dov C, Guigó R, et al. CPEB1 coordinates alternative 3′-UTR formation with translational regulation. Nature. 2013; 495:121–125.
Article32. Jenal M, Elkon R, Loayza-Puch F, van Haaften G, Kühn U, Menzies FM, et al. The poly(A)-binding protein nuclear 1 suppresses alternative cleavage and polyadenylation sites. Cell. 2012; 149:538–553.
Article33. Yeh HS, Zhang W, Yong J. Analyses of alternative polyadenylation: from old school biochemistry to high-throughput technologies. BMB Rep. 2017; 50:201–207.
Article34. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010; 79:351–379.
Article35. Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science. 2008; 320:1643–1647.
Article36. Boutet SC, Cheung TH, Quach NL, Liu L, Prescott SL, Edalati A, et al. Alternative polyadenylation mediates microRNA regulation of muscle stem cell function. Cell Stem Cell. 2012; 10:327–336.
Article37. Ciolli Mattioli C, Rom A, Franke V, Imami K, Arrey G, Terne M, et al. Alternative 3′ UTRs direct localization of functionally diverse protein isoforms in neuronal compartments. Nucleic Acids Res. 2019; 47:2560–2573.
Article38. Berkovits BD, Mayr C. Alternative 3′ UTRs act as scaffolds to regulate membrane protein localization. Nature. 2015; 522:363–367.
Article39. Mayr C. Evolution and biological roles of alternative 3'UTRs. Trends Cell Biol. 2016; 26:227–237.
Article40. Hoque M, Ji Z, Zheng D, Luo W, Li W, You B, et al. Analysis of alternative cleavage and polyadenylation by 3′ region extraction and deep sequencing. Nat Methods. 2013; 10:133–139.
Article41. Rogers J, Early P, Carter C, Calame K, Bond M, Hood L, et al. Two mRNAs with different 3′ ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980; 20:303–312.
Article42. O'Loghlen A, González VM, Piñeiro D, Pérez-Morgado MI, Salinas M, Martín ME. Identification and molecular characterization of Mnk1b, a splice variant of human MAP kinase-interacting kinase Mnk1. Exp Cell Res. 2004; 299:343–355.43. Lee SH, Singh I, Tisdale S, Abdel-Wahab O, Leslie CS, Mayr C. Widespread intronic polyadenylation inactivates tumour suppressor genes in leukaemia. Nature. 2018; 561:127–131.
Article44. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007; 12:9–22.
Article45. Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007; 8:774–785.
Article46. Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005; 12 Suppl 2:1509–1518.
Article47. Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011; 4:51.
Article48. Liu J, Ma K, Gao M, Zhang X, Liu B. The activation of mTOR pathway induced by inflammation accelerates the progression of atherosclerosis in hemodialysis patients. Int J Cardiol. 2011; 152:S5–S6.
Article49. Jones RG, Pearce EJ. MenTORing immunity: mTOR signaling in the development and function of tissue-resident immune cells. Immunity. 2017; 46:730–742.
Article50. Kurdi A, Martinet W, De Meyer GR. mTOR inhibition and cardiovascular diseases: dyslipidemia and atherosclerosis. Transplantation. 2018; 102:S44–S46.51. Xie J, Wang X, Proud CG. mTOR inhibitors in cancer therapy. F1000Res. 2016; 5:2078.
Article52. Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005; 433:477–480.
Article53. Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012; 485:109–113.
Article54. Mayer C, Zhao J, Yuan X, Grummt I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 2004; 18:423–434.
Article55. Ben-Sahra I, Hoxhaj G, Ricoult SJ, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016; 351:728–733.
Article56. Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010; 39:171–183.
Article57. Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature. 2007; 450:736–740.
Article58. Chang JW, Zhang W, Yeh HS, de Jong EP, Jun S, Kim KH, et al. mRNA 3′-UTR shortening is a molecular signature of mTORC1 activation. Nat Commun. 2015; 6:7218.
Article59. Shi Y. Alternative polyadenylation: new insights from global analyses. RNA. 2012; 18:2105–2117.
Article60. Fox-Walsh K, Davis-Turak J, Zhou Y, Li H, Fu XD. A multiplex RNA-seq strategy to profile poly(A+) RNA: application to analysis of transcription response and 3′ end formation. Genomics. 2011; 98:266–271.
Article61. Chang JW, Zhang W, Yeh HS, Park M, Yao C, Shi Y, et al. An integrative model for alternative polyadenylation, IntMAP, delineates mTOR-modulated endoplasmic reticulum stress response. Nucleic Acids Res. 2018; 46:5996–6008.
Article62. Passacantilli I, Frisone P, De Paola E, Fidaleo M, Paronetto MP. hnRNPM guides an alternative splicing program in response to inhibition of the PI3K/AKT/mTOR pathway in Ewing sarcoma cells. Nucleic Acids Res. 2017; 45:12270–12284.
Article63. Lee G, Zheng Y, Cho S, Jang C, England C, Dempsey JM, et al. Post-transcriptional regulation of de novo lipogenesis by mTORC1-S6K1-SRPK2 signaling. Cell. 2017; 171:1545–1558.e18.
Article64. Chang JW, Yeh HS, Park M, Erber L, Sun J, Cheng S, et al. mTOR-regulated U2af1 tandem exon splicing specifies transcriptome features for translational control. Nucleic Acids Res. 2019; 47:10373–10387.
Article65. Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012; 13:227–232.
Article66. Reyes A, Huber W. Alternative start and termination sites of transcription drive most transcript isoform differences across human tissues. Nucleic Acids Res. 2018; 46:582–592.
Article67. Lerner T, Papavasiliou FN, Pecori R. RNA editors, cofactors, and mRNA targets: an overview of the C-to-U RNA editing machinery and its implication in human disease. Genes (Basel). 2018; 10:13.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- AR-mTOR-SRF Axis Regulates HMMR Expression in Human Prostate Cancer Cells
- Post-transcriptional Regulation of NK Cell Activation
- Temporal changes in mammalian target of rapamycin (mTOR) and phosphorylated-mTOR expressions in the hippocampal CA1 region of rat with vascular dementia
- Regulation of fatty acid synthase at transcriptional and post-transcriptional levels in rat liver
- Coordinated transcriptional regulation of calmegin, a testis-specific molecular chaperon, by histone deacetylase and CpG methyltransferase