J Neurocrit Care.
2019 Dec;12(2):74-84. 10.18700/jnc.190109.
Cefepime-induced neurotoxicity
- Affiliations
-
- 1Department of Neurology, Yeungnam University College of Medicine, Daegu, Republic of Korea. sejinmayo@ynu.ac.kr
- KMID: 2470468
- DOI: http://doi.org/10.18700/jnc.190109
Abstract
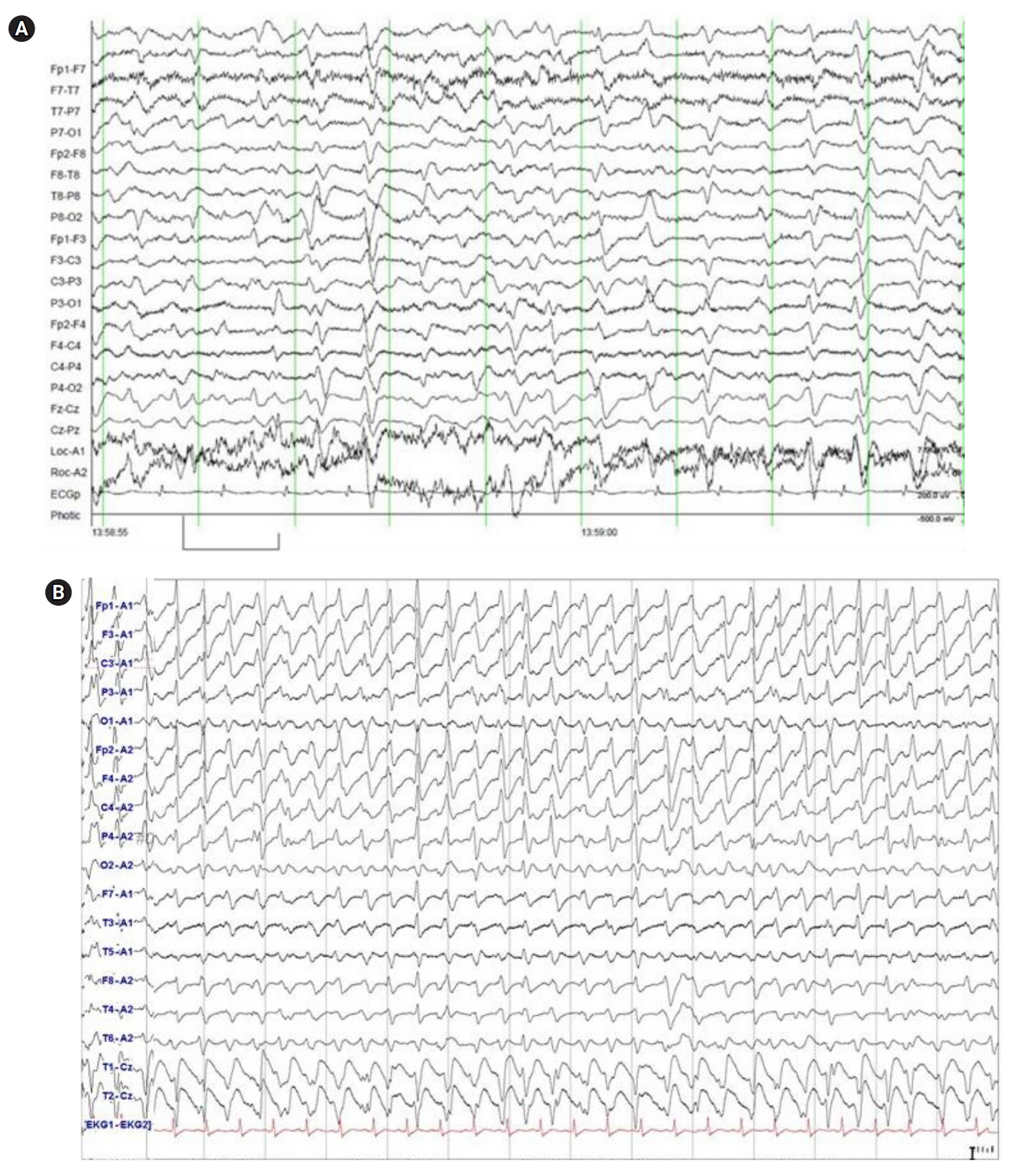
- Cefepime, a widely used fourth-generation cephalosporin, has been reported to cause neurotoxicity because it crosses the blood-brain barrier. Although cefepime-induced neurotoxicity (CIN) occurs in patients with renal dysfunction administered a high dosage, CIN has also been reported in patients with normal renal function administered the appropriate dosage. CIN is characterized by toxic encephalopathy and electroencephalography abnormalities, such as triphasic wave, currently renamed as generalized periodic discharge (GPD) with triphasic morphology, and nonconvulsive status epilepticus (NCSE). Toxic encephalopathy appears 2 to 6 days after cefepime administration and disappears 3 days after discontinuation of cefepime. Electroencephalography abnormalities in most reported cases are GPD with triphasic morphology rather than NCSE. CIN is reversible in most cases if early detection and discontinuation of cefepime is possible, which is the only definitive treatment; however, anticonvulsant therapy is unnecessary except for patients with convulsive seizures or definite NCSE. Emergent hemodialysis may also be helpful in life-threatening situations.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Cephalosporin-induced encephalopathy in patients with hematologic malignancies: a significant concern
Young Seob Park, Min Kyoung Kim, Kyung Hee Lee, Sung Ae Koh, Ji Yoon Jung, Byeong Il Jang, Se-Jin Lee
J Yeungnam Med Sci. 2023;40(Suppl):S137-S141. doi: 10.12701/jyms.2023.00864.
Reference
-
1. Neu HC. Safety of cefepime: a new extended-spectrum parenteral cephalosporin. Am J Med. 1996; 100(6A):68S–75S.
Article2. Wong KM, Chan WK, Chan YH, Li CS. Cefepime-related neurotoxicity in a haemodialysis patient. Nephrol Dial Transplant. 1999; 14:2265–6.
Article3. Jallon P, Fankhauser L, Du Pasquier R, Coeytaux A, Picard F, Hefft S, et al. Severe but reversible encephalopathy associated with cefepime. Neurophysiol Clin. 2000; 30:383–6.
Article4. Garces EO, Andrade de Anzambuja MF, da Silva D, Bragatti JA, Jacoby T, Saldanha Thomé F. Renal failure is a risk factor for cefepime-induced encephalopathy. J Nephrol. 2008; 21:526–34.5. Thabet F, Al Maghrabi M, Al Barraq A, Tabarki B. Cefepime-induced nonconvulsive status epilepticus: case report and review. Neurocrit Care. 2009; 10:347–51.
Article6. Fugate JE, Kalimullah EA, Hocker SE, Clark SL, Wijdicks EF, Rabinstein AA. Cefepime neurotoxicity in the intensive care unit: a cause of severe, underappreciated encephalopathy. Crit Care. 2013; 17:R264.
Article7. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015; 85:1332–41.8. Bhattacharyya S, Darby RR, Raibagkar P, Gonzalez Castro LN, Berkowitz AL. Antibiotic-associated encephalopathy. Neurology. 2016; 86:963–71.
Article9. Appa AA, Jain R, Rakita RM, Hakimian S, Pottinger PS. Characterizing cefepime neurotoxicity: a systematic review. Open Forum Infect Dis. 2017; 4:ofx170.
Article10. Payne LE, Gagnon DJ, Riker RR, Seder DB, Glisic EK, Morris JG, et al. Cefepime-induced neurotoxicity: a systematic review. Crit Care. 2017; 21:276.
Article11. Cho KH. Antibiotics induced seizures and encephalopathy. J Neurocrit Care. 2018; 11:1–6.
Article12. Saurina A, Vera M, Pou M, López Pedret J, Darnell A, Campistol JM, et al. Non-convulsive status epilepticus secondary to adjusted cefepime doses in patients with chronic renal failure. Nefrologia. 2000; 20:554–8.13. Martínez-Rodríguez JE, Barriga FJ, Santamaria J, Iranzo A, Pareja JA, Revilla M, et al. Nonconvulsive status epilepticus associated with cephalosporins in patients with renal failure. Am J Med. 2001; 111:115–9.
Article14. Ferrara N, Abete P, Giordano M, Ferrara P, Carnovale V, Leosco D, et al. Neurotoxicity induced by cefepime in a very old hemodialysis patient. Clin Nephrol. 2003; 59:388–90.
Article15. De Silva DA, Pan AB, Lim SH. Cefepime-induced encephalopathy with triphasic waves in three Asian patients. Ann Acad Med Singapore. 2007; 36:450–1.16. Gangireddy VG, Mitchell LC, Coleman T. Cefepime neurotoxicity despite renal adjusted dosing. Scand J Infect Dis. 2011; 43:827–9.
Article17. Nakagawa R, Sato K, Uesaka Y, Mitsuki T, Kondo K, Wake A, et al. Cefepime-induced encephalopathy in end-stage renal disease patients. J Neurol Sci. 2017; 376:123–8.
Article18. Li HT, Lee CH, Wu T, Cheng MY, Tseng WJ, Chang CW, et al. Clinical, electroencephalographic features and prognostic factors of cefepime-induced neurotoxicity: a retrospective study. Neurocrit Care. 2019; 31:329–37.
Article19. Capparelli FJ, Diaz MF, Hlavnika A, Wainsztein NA, Leiguarda R, Del Castillo ME. Cefepime- and cefixime-induced encephalopathy in a patient with normal renal function. Neurology. 2005; 65:1840.
Article20. Fernández-Torre JL, Martínez-Martínez M, González-Rato J, Maestro I, Alonso I, Rodrigo E, et al. Cephalosporin-induced nonconvulsive status epilepticus: clinical and electroencephalographic features. Epilepsia. 2005; 46:1550–2.
Article21. Maganti R, Jolin D, Rishi D, Biswas A. Nonconvulsive status epilepticus due to cefepime in a patient with normal renal function. Epilepsy Behav. 2006; 8:312–4.
Article22. Meillier A, Rahimian D. Cefepime-induced encephalopathy with normal renal function. Oxf Med Case Reports. 2016; 2016:118–20.
Article23. U.S. Food and Drug Administration. FDA Drug Safety Communication: cefepime and risk of seizure in patients not receiving dosage adjustments for kidney impairment [Internet]. Silver Spring (MD): FDA;2016. [cited 2019 Dec 16]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm309661.htm.24. Ugai T, Morisaki K, Tsuda K, Sugihara H, Nishida Y, Yamakura M, et al. Cefepime-induced encephalopathy in patients with haematological malignancies: clinical features and risk factors. Scand J Infect Dis. 2014; 46:272–9.
Article25. Kang JK, Kim SB. Neurotoxicity by cefepime: case-control study. J Neurocrit Care. 2014; 7:104–10.
Article26. Lamoth F, Buclin T, Pascual A, Vora S, Bolay S, Decosterd LA, et al. High cefepime plasma concentrations and neurological toxicity in febrile neutropenic patients with mild impairment of renal function. Antimicrob Agents Chemother. 2010; 54:4360–7.
Article27. Huwyler T, Lenggenhager L, Abbas M, Ing Lorenzini K, Hughes S, Huttner B, et al. Cefepime plasma concentrations and clinical toxicity: a retrospective cohort study. Clin Microbiol Infect. 2017; 23:454–9.
Article28. Boschung-Pasquier L, Atkinson A, Kastner LK, Banholzer S, Haschke M, Buetti N, et al. Cefepime neurotoxicity: thresholds and risk factors: a retrospective cohort study. Clin Microbiol Infect. 2019; July. 5. https://doi.org/10.1016/j.cmi.2019.06.028.
Article29. Lacroix C, Kheloufi F, Montastruc F, Bennis Y, Pizzoglio V, Micallef J. Serious central nervous system side effects of cephalosporins: a national analysis of serious reports registered in the French Pharmacovigilance Database. J Neurol Sci. 2019; 398:196–201.
Article30. U.S. Food and Drug Administration. Drug approval package: Maxipime (cefepime hydrochloride) for injection [Internet]. Silver Spring (MD): FDA;2002. [cited 2019 Dec 16]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/050679s031lbl.pdf.31. Neftel KA, Cerny A, Cottagnoud P. Cephalosporins. In : Dukes MNG, editor. Meyler’s Side Effects of Drugs. 13th ed. Amsterdam: Elsevier;1996. p. 711–8.32. Barbhaiya RH, Forgue ST, Gleason CR, Knupp CA, Pittman KA, Weidler DJ, et al. Safety, tolerance, and pharmacokinetic evaluation of cefepime after administration of single intravenous doses. Antimicrob Agents Chemother. 1990; 34:1118–22.
Article33. Barbhaiya RH, Knupp CA, Forgue ST, Matzke GR, Guay DR, Pittman KA. Pharmacokinetics of cefepime in subjects with renal insufficiency. Clin Pharmacol Ther. 1990; 48:268–76.
Article34. Barbhaiya RH, Knupp CA, Pfeffer M, Zaccardelli D, Dukes GM, Mattern W, et al. Pharmacokinetics of cefepime in patients undergoing continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1992; 36:1387–91.
Article35. U.S. Food and Drug Administration. Cefepime (marketed as Maxipime) update of ongoing safety review [Internet]. Silver Spring (MD): FDA;2009. [cited 2019 Dec 16]. Available from: https://www.fda.gov/.36. Sugimoto M, Uchida I, Mashimo T, Yamazaki S, Hatano K, Ikeda F, et al. Evidence for the involvement of GABA(A) receptor blockade in convulsions induced by cephalosporins. Neuropharmacology. 2003; 45:304–14.
Article37. De Sarro A, Ammendola D, Zappala M, Grasso S, De Sarro GB. Relationship between structure and convulsant properties of some beta-lactam antibiotics following intracerebroventricular microinjection in rats. Antimicrob Agents Chemother. 1995; 39:232–7.
Article38. Grill MF, Maganti R. Cephalosporin-induced neurotoxicity: clinical manifestations, potential pathogenic mechanisms, and the role of electroencephalographic monitoring. Ann Pharmacother. 2008; 42:1843–50.
Article39. Kim SH, Nam YH, Jeon DS, Lee HW, Nam HJ, Lee SK. Cefepime-induced encephalopathy without renal impairment. Allergy Asthma Respir Dis. 2014; 2:213–7.
Article40. Park HM, Noh Y, Yang JW, Shin DH, Lee YB. Cefepime-induced non-convulsive status epilepticus in a patient with normal renal function. J Epilepsy Res. 2016; 6:97–9.
Article41. Baek SD, Park SJ, Baek CH, Koo TY, Kang JK, Kim SB. Neurotoxicity induced by cefepime in a patient with minimal change disease. Korean J Nephrol. 2010; 29:796–801.42. Lee JY, Kang KP, Kim W, Park SK, Lee S. An overlooked cause of impaired consciousness in a hemodialysis patient. Korean J Intern Med. 2012; 27:367.
Article43. Triplett JD, Lawn ND, Chan J, Dunne JW. Cephalosporin-related neurotoxicity: metabolic encephalopathy or non-convulsive status epilepticus? J Clin Neurosci. 2019; 67:163–6.
Article44. Fishman RA. Blood-brain and CSF barriers to penicillin and related organic acids. Arch Neurol. 1966; 15:113–24.
Article45. Chatellier D, Jourdain M, Mangalaboyi J, Ader F, Chopin C, Derambure P, et al. Cefepime-induced neurotoxicity: an underestimated complication of antibiotherapy in patients with acute renal failure. Intensive Care Med. 2002; 28:214–7.
Article46. Durand-Maugard C, Lemaire-Hurtel AS, Gras-Champel V, Hary L, Maizel J, Prud'homme-Bernardy A, et al. Blood and CSF monitoring of cefepime-induced neurotoxicity: nine case reports. J Antimicrob Chemother. 2012; 67:1297–9.
Article47. Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013; 19:1584–96.
Article48. Abanades S, Nolla J, Rodríguez-Campello A, Pedro C, Valls A, Farré M. Reversible coma secondary to cefepime neurotoxicity. Ann Pharmacother. 2004; 38:606–8.
Article49. Ryu JA, Lee SM, Kim JI, Lee GH, Lee CM, Song YM, et al. Cefepime-induced reversible encephalopathy with triphasic waves in patients with impaired renal function. J Korean Epilepsy Soc. 2009; 13:15–8.50. Seyler L, Cotton F, Taccone FS, De Backer D, Macours P, Vincent JL, et al. Recommended β-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit Care. 2011; 15:R137.
Article51. Lam S, Gomolin IH. Cefepime neurotoxicity: case report, pharmacokinetic considerations, and literature review. Pharmacotherapy. 2006; 26:1169–74.
Article52. Rhodes NJ, Kuti JL, Nicolau DP, Neely MN, Nicasio AM, Scheetz MH. An exploratory analysis of the ability of a cefepime trough concentration greater than 22 mg/L to predict neurotoxicity. J Infect Chemother. 2016; 22:78–83.53. Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society's standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013; 30:1–27.54. Hartshorn JA, Foreman B. Generalized periodic discharges with triphasic morphology. J Neurocrit Care. 2019; 12:1–8.
Article55. Lee S. Pregabalin intoxication-induced encephalopathy with triphasic waves. Epilepsy Behav. 2012; 25:170–3.
Article56. Kifune A, Kubota F, Shibata N, Akata T, Kikuchi S. Valproic acid-induced hyperammonemic encephalopathy with triphasic waves. Epilepsia. 2000; 41:909–12.
Article57. Dike GL. Triphasic waves in serotonin syndrome. J Neurol Neurosurg Psychiatry. 1997; 62:200.
Article58. Hormes JT, Benarroch EE, Rodriguez M, Klass DW. Periodic sharp waves in baclofen-induced encephalopathy. Arch Neurol. 1988; 45:814–5.
Article59. Kaplan PW, Birbeck G. Lithium-induced confusional states: nonconvulsive status epilepticus or triphasic encephalopathy? Epilepsia. 2006; 47:2071–4.
Article60. Neufeld MY. Periodic triphasic waves in levodopa-induced encephalopathy. Neurology. 1992; 42:444–6.
Article61. Lancman ME, Marks S, Mahmood K, Lansen T. Atypical triphasic waves associated with the use of pentobarbital. Electroencephalogr Clin Neurophysiol. 1997; 102:175–7.
Article62. de Borchgrave V, Lienard F, Willemart T, van Rijckevorsel K. Clinical and EEG findings in six patients with altered mental status receiving tiagabine therapy. Epilepsy Behav. 2003; 4:326–37.
Article63. Vulliemoz S, Iwanowski P, Landis T, Jallon P. Levetiracetam accumulation in renal failure causing myoclonic encephalopathy with triphasic waves. Seizure. 2009; 18:376–8.
Article64. Kaplan PW, Sutter R. Affair with triphasic waves-their striking presence, mysterious significance, and cryptic origins: what are they? J Clin Neurophysiol. 2015; 32:401–5.65. Beniczky S, Hirsch LJ, Kaplan PW, Pressler R, Bauer G, Aurlien H, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013; 54 Suppl 6:28–9.
Article66. Trinka E, Leitinger M. Which EEG patterns in coma are nonconvulsive status epilepticus? Epilepsy Behav. 2015; 49:203–22.
Article67. Bauer G, Trinka E. Nonconvulsive status epilepticus and coma. Epilepsia. 2010; 51:177–90.
Article68. Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996; 47:83–9.69. Lee SJ. A comatose patient with continuous generalized 3 Hz spike-and-wave discharges after cardiac arrest. J Korean Neurol Assoc. 2015; 33:209–12.
Article70. Bragatti JA, Rossato R, Ziomkowski S, Kliemann FA. Cefepime-induced encephalopathy: clinical and electroencephalographic features in seven patients. Arq Neuropsiquiatr. 2005; 63:87–92.71. Sonck J, Laureys G, Verbeelen D. The neurotoxicity and safety of treatment with cefepime in patients with renal failure. Nephrol Dial Transplant. 2008; 23:966–70.
Article72. Lin CJ, Chen SP, Wang SJ, Fuh JL. Cefepime-related encephalopathy in peritoneal dialysis patients. J Chin Med Assoc. 2011; 74:87–90.
Article73. Shirota Y, Ohtomo R, Hanajima R, Terao Y, Tsutsumi R, Tsuji S. Severely abnormal electroencephalogram in two patients who were treated with cefepime. Rinsho Shinkeigaku. 2012; 52:356–9.
Article74. Mani LY, Kissling S, Viceic D, Vogt B, Burnier M, Buclin T, et al. Intermittent hemodialysis treatment in cefepime-induced neurotoxicity: case report, pharmacokinetic modeling, and review of the literature. Hemodial Int. 2015; 19:333–43.
Article75. Toda Y, Yamazaki M, Ota T, Fujisawa Y, Kimura K. A case of cefepime encephalopathy, being difficult to distinguish from non-convulsive status epilepticus during the treatment of bacterial meningitis. Rinsho Shinkeigaku. 2016; 56:678–83.
Article76. Tchapyjnikov D, Luedke MW. Cefepime-induced encephalopathy and nonconvulsive status epilepticus: dispelling an artificial dichotomy. Neurohospitalist. 2019; 9:100–4.
Article77. Dixit S, Kurle P, Buyan-Dent L, Sheth RD. Status epilepticus associated with cefepime. Neurology. 2000; 54:2153–5.
Article78. Chow KM, Wang AY, Hui AC, Wong TY, Szeto CC, Li PK. Nonconvulsive status epilepticus in peritoneal dialysis patients. Am J Kidney Dis. 2001; 38:400–5.
Article79. Plensa E, Gallardo E, Ribera JM, Batlle M, Oriol A, Costa J. Nonconvulsive status epilepticus associated with cefepime in a patient undergoing autologous stem cell transplantation. Bone Marrow Transplant. 2004; 33:119–20.
Article80. Primavera A, Cocito L, Audenino D. Nonconvulsive status epilepticus during cephalosporin therapy. Neuropsychobiology. 2004; 49:218–22.
Article81. Chang YM. Cefepime-induced nonconvulsive status epilepticus as a cause of confusion in an elderly patient. J Formos Med Assoc. 2015; 114:290–1.
Article82. Suarez-de-la-Rica A, Hernández-Gancedo C, López-Tofiño A, Maseda E, Gilsanz F. Severe cefepime-induced status epilepticus treated with haemofiltration. Rev Esp Anestesiol Reanim. 2016; 63:353–6.
Article83. Bora I, Demir AB, Uzun P. Nonconvulsive status epilepticus cases arising in connection with cephalosporins. Epilepsy Behav Case Rep. 2016; 6:23–7.
Article84. Anuhya V, Kunder SK, Madhyastha S, Nayak V, Acharya RV, Ramamoorthi K, et al. Looking beyond the obvious: cefepime-induced nonconvulsive status epilepticus. J Pharmacol Pharmacother. 2017; 8:145–7.85. Fountain NB, Waldman WA. Effects of benzodiazepines on triphasic waves: implications for nonconvulsive status epilepticus. J Clin Neurophysiol. 2001; 18:345–52.86. Brigo F, Storti M. Triphasic waves. Am J Electroneurodiagnostic Technol. 2011; 51:16–25.
Article87. Malone RS, Fish DN, Abraham E, Teitelbaum I. Pharmacokinetics of cefepime during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother. 2001; 45:3148–55.
Article88. Kim A, Kim JE, Paek YM, Hong KS, Cho YJ, Cho JY, et al. Cefepime- induced non-convulsive status epilepticus (NCSE). J Epilepsy Res. 2013; 3:39–41.
Article89. Kwon J, Choi JY, Bae EK. Cefepime-induced aphasic status epilepticus mimicking acute stroke. J Epilepsy Res. 2014; 4:85–7.
Article90. Lee GH. Cefepime induced non-convulsive status epilepticus (NCSE). J Pusan Natl Univ Hosp. 2014; 36:185–9.91. Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis. 2007; 7:338–48.
Article92. Kim PW, Wu YT, Cooper C, Rochester G, Valappil T, Wang Y, et al. Meta-analysis of a possible signal of increased mortality associated with cefepime use. Clin Infect Dis. 2010; 51:381–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cefepime Neurotoxicity in Patients with Renal Insufficiency
- Neurotoxicity Induced by Cefepime in a Patient with Minimal Change Disease
- Cefepime-induced neurotoxicity after flap surgery: a rare case report
- Cefepime-Induced Reversible Encephalopathy with Triphasic Waves in Patients with Impaired Renal Function
- Cefepime-induced nonconvulsive status epilepticus in a hemodialysis patient