Allergy Asthma Respir Dis.
2020 Jan;8(1):3-8. 10.4168/aard.2020.8.1.3.
Cystic fibrosis lung disease: Current perspectives
- Affiliations
-
- 1Department of Pediatrics, Dong-A University College of Medicine, Busan, Korea. jina1477@dau.ac.kr
- KMID: 2469112
- DOI: http://doi.org/10.4168/aard.2020.8.1.3
Abstract
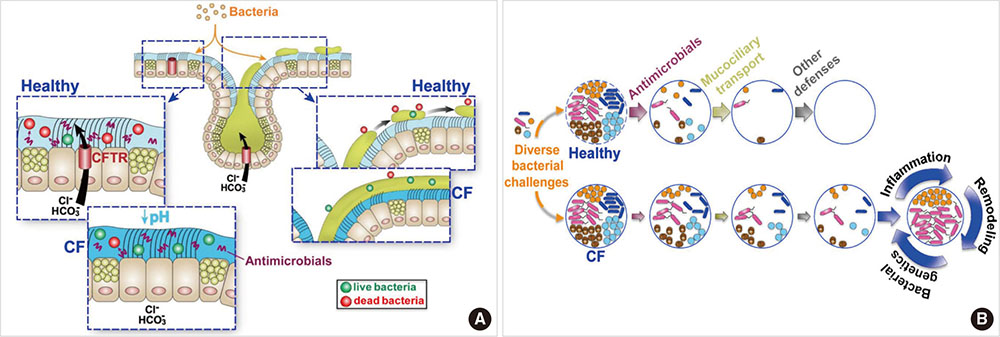
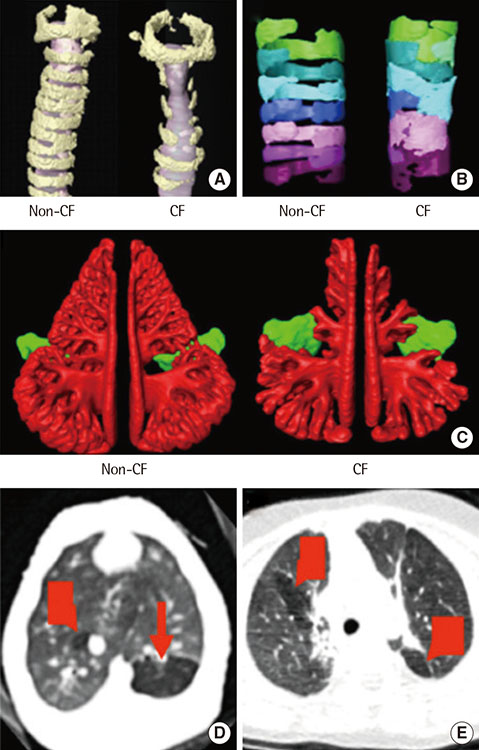
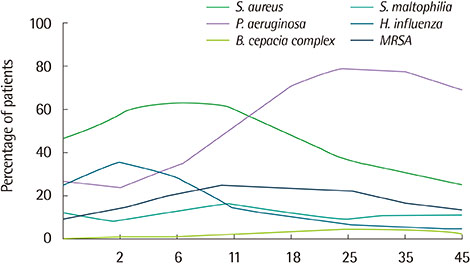
- Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). These mutations alter the synthesis, processing, function, or half-life of CFTR, the main chloride channel expressed in the apical membrane of epithelial cells in the airway, intestine, pancreas, and reproductive tract. Lung disease is the most critical manifestation of CF. It is characterized by airway obstruction, infection, and inflammation that lead to fatal tissue destruction, which causes most CF morbidity and mortality. This article reviews the pathophysiology of CF, recent animal models, and current treatment of CF.
Keyword
MeSH Terms
-
Airway Obstruction
Chloride Channels
Cystic Fibrosis Transmembrane Conductance Regulator
Cystic Fibrosis*
Epithelial Cells
Epithelial Sodium Channels
Half-Life
Inflammation
Intestines
Lung Diseases*
Lung*
Membranes
Models, Animal
Mortality
Pancreas
Chloride Channels
Cystic Fibrosis Transmembrane Conductance Regulator
Epithelial Sodium Channels
Figure
Reference
-
1. Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989; 245:1066–1073.
Article2. Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic fibrosis. In : Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B, editors. The metabolic and molecular basis of inherited disease. 8th ed. New York: McGraw-Hill;2001. p. 5121–5189.3. Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015; 372:351–362.
Article4. Moon HR, Ko TS, Ko YY, Choi JH, Kim YC. Cystic fibrosis--a case presented with recurrent bronchiolitis in infancy in a Korean male infant. J Korean Med Sci. 1988; 3:157–162.
Article5. Ahn KM, Park HY, Lee JH, Lee MG, Kim JH, Kang IJ, et al. Cystic fibrosis in Korean children:a case report identified by a quantitative pilocarpine iontophoresis sweat test and genetic analysis. J Korean Med Sci. 2005; 20:153–157.
Article6. Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007; 58:157–170.
Article7. Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med. 2012; 18:509–519.
Article8. Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, et al. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991; 253:202–205.
Article9. Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008; 372:415–417.
Article10. Hobbs CA, Da Tan C, Tarran R. Does epithelial sodium channel hyperactivity contribute to cystic fibrosis lung disease? J Physiol. 2013; 591:4377–4387.
Article11. Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, et al. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010; 143:911–923.
Article12. Tuggle KL, Birket SE, Cui X, Hong J, Warren J, Reid L, et al. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS One. 2014; 9:e91253.
Article13. Sun X, Olivier AK, Liang B, Yi Y, Sui H, Evans TI, et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am J Respir Cell Mol Biol. 2014; 50:502–512.
Article14. Itani OA, Chen JH, Karp PH, Ernst S, Keshavjee S, Parekh K, et al. Human cystic fibrosis airway epithelia have reduced Cl- conductance but not increased Na+ conductance. Proc Natl Acad Sci U S A. 2011; 108:10260–10265.
Article15. Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012; 487:109–113.
Article16. Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014; 345:818–822.
Article17. Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008; 321:1837–1841.
Article18. Bonvin E, Le Rouzic P, Bernaudin JF, Cottart CH, Vandebrouck C, Crié A, et al. Congenital tracheal malformation in cystic fibrosis transmembrane conductance regulator-deficient mice. J Physiol. 2008; 586:3231–3243.
Article19. Meyerholz DK, Stoltz DA, Namati E, Ramachandran S, Pezzulo AA, Smith AR, et al. Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med. 2010; 182:1251–1261.
Article20. Chang EH, Pezzulo AA, Meyerholz DK, Potash AE, Wallen TJ, Reznikov LR, et al. Sinus hypoplasia precedes sinus infection in a porcine model of cystic fibrosis. Laryngoscope. 2012; 122:1898–1905.
Article21. Adam RJ, Michalski AS, Bauer C, Abou Alaiwa MH, Gross TJ, Awadalla MS, et al. Air trapping and airflow obstruction in newborn cystic fibrosis piglets. Am J Respir Crit Care Med. 2013; 188:1434–1441.
Article22. Fischer AJ, Singh SB, Adam RJ, Stoltz DA, Baranano CF, Kao S, et al. Tracheomalacia is associated with lower FEV1 and Pseudomonas acquisition in children with CF. Pediatr Pulmonol. 2014; 49:960–970.
Article23. Schmidt BZ, Haaf JB, Leal T, Noel S. Cystic fibrosis transmembrane conductance regulator modulators in cystic fibrosis: current perspectives. Clin Pharmacol. 2016; 8:127–140.24. Haardt M, Benharouga M, Lechardeur D, Kartner N, Lukacs GL. C-terminal truncations destabilize the cystic fibrosis transmembrane conductance regulator without impairing its biogenesis. A novel class of mutation. J Biol Chem. 1999; 274:21873–21877.
Article25. Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993; 73:1251–1254.
Article26. Varga K, Goldstein RF, Jurkuvenaite A, Chen L, Matalon S, Sorscher EJ, et al. Enhanced cell-surface stability of rescued DeltaF508 cystic fibrosis transmembrane conductance regulator (CFTR) by pharmacological chaperones. Biochem J. 2008; 410:555–564.
Article27. Cystic Fibrosis Foundation. 2013 CF Foundation Patient Registry Annual Data Report to the Center Directors [Internet]. Bethesda (MD): Cystic Fibrosis Foundation;c2014. cited 2019 Mar 19. Available from: https://www.cff.org/2013_CFF_Annual_Data_Report_to_the_Center_Directors.pdf.28. McCormack J, Bell S, Senini S, Walmsley K, Patel K, Wainwright C, et al. Daily versus weekly azithromycin in cystic fibrosis patients. Eur Respir J. 2007; 30:487–495.
Article29. Lands LC, Milner R, Cantin AM, Manson D, Corey M. High-dose ibuprofen in cystic fibrosis: Canadian safety and effectiveness trial. J Pediatr. 2007; 151:249–254.
Article30. Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJ, et al. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005; 115:2564–2571.
Article31. Van Goor F, Straley KS, Cao D, González J, Hadida S, Hazlewood A, et al. Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006; 290:L1117–L1130.32. Dhooghe B, Haaf JB, Noel S, Leal T. Strategies in early clinical development for the treatment of basic defects of cystic fibrosis. Expert Opin Investig Drugs. 2016; 25:423–436.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cystic Fibrosis: Case Report
- A Case of Mycobacterium abscessus Lung Disease in a Patient with Cystic Fibrosis
- Cystic fibrosis in a female adolescent carrying c.263T>G (p.Leu88X) and c.2977G>T (p.Asp993Tyr) mutation
- Radiologic Findings of Cystic Fibrosis in a Korean Child at Follow Up Study: Case Report
- Bronchiectasis