J Korean Med Sci.
2016 Oct;31(10):1586-1594. 10.3346/jkms.2016.31.10.1586.
Eukaryotic Translation Initiation Factor 3a (eIF3a) Promotes Cell Proliferation and Motility in Pancreatic Cancer
- Affiliations
-
- 1General Surgery Department, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, China.
- 2Department of Neurosurgery, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, China. hughjin@126.com
- KMID: 2468249
- DOI: http://doi.org/10.3346/jkms.2016.31.10.1586
Abstract
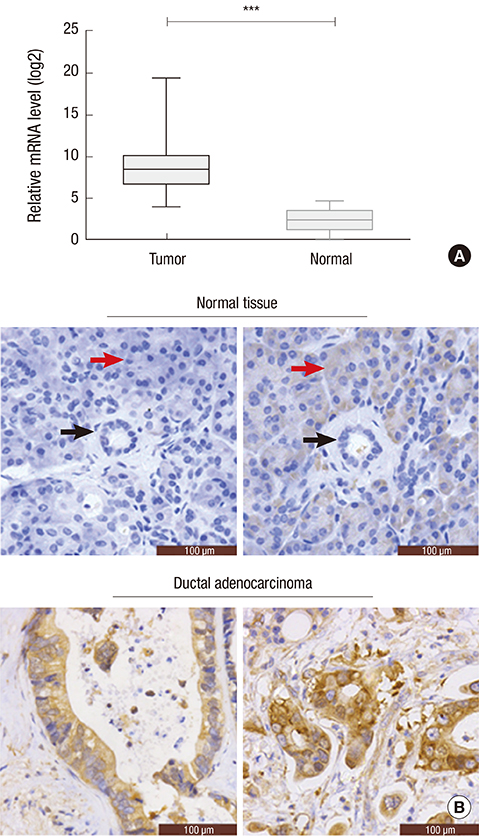
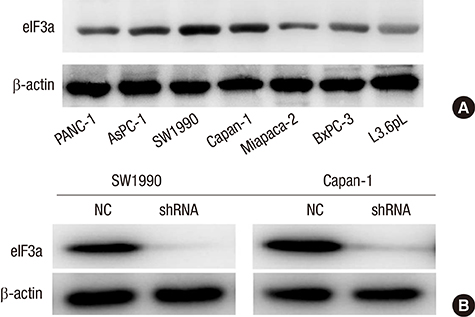
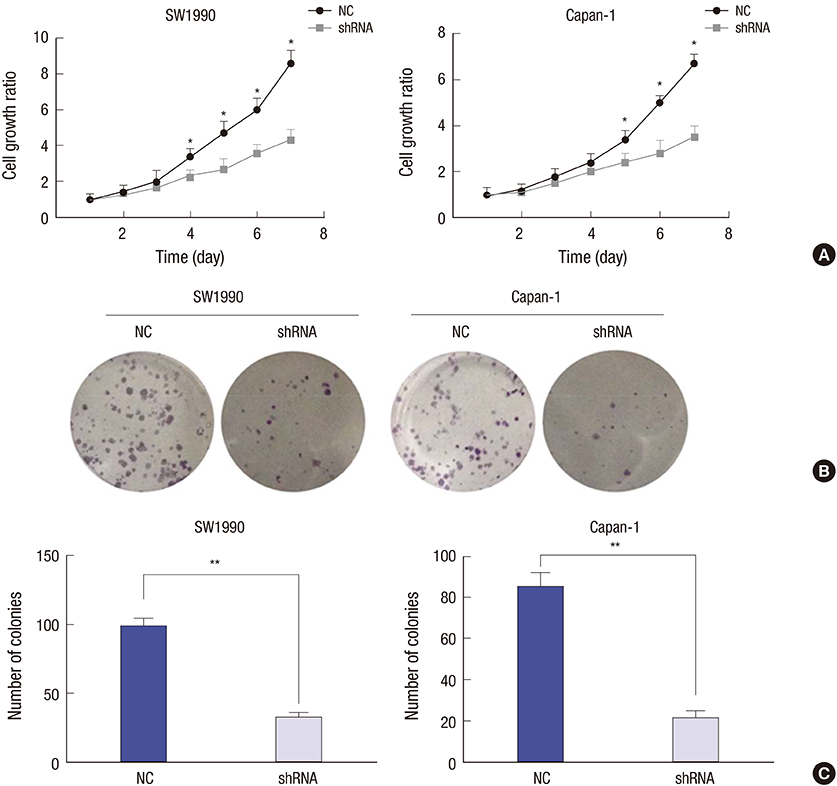
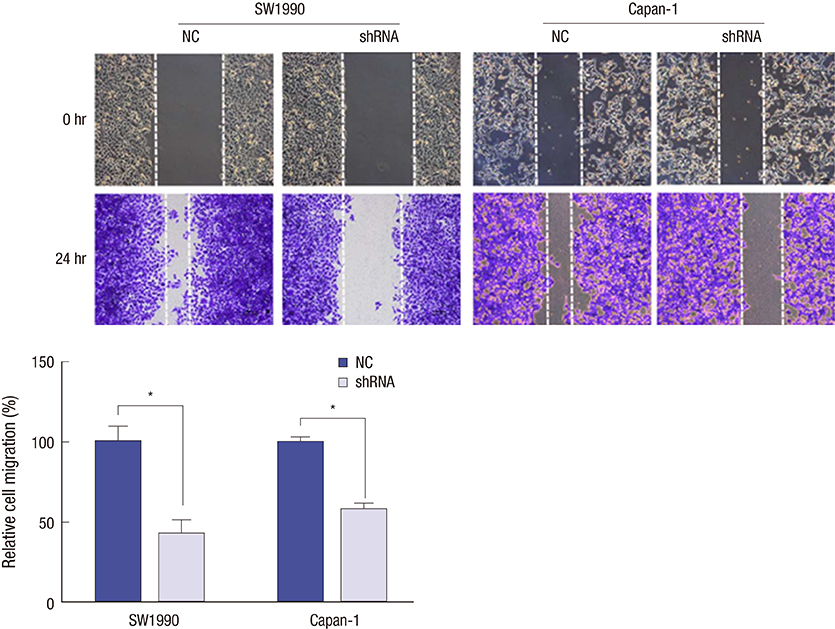
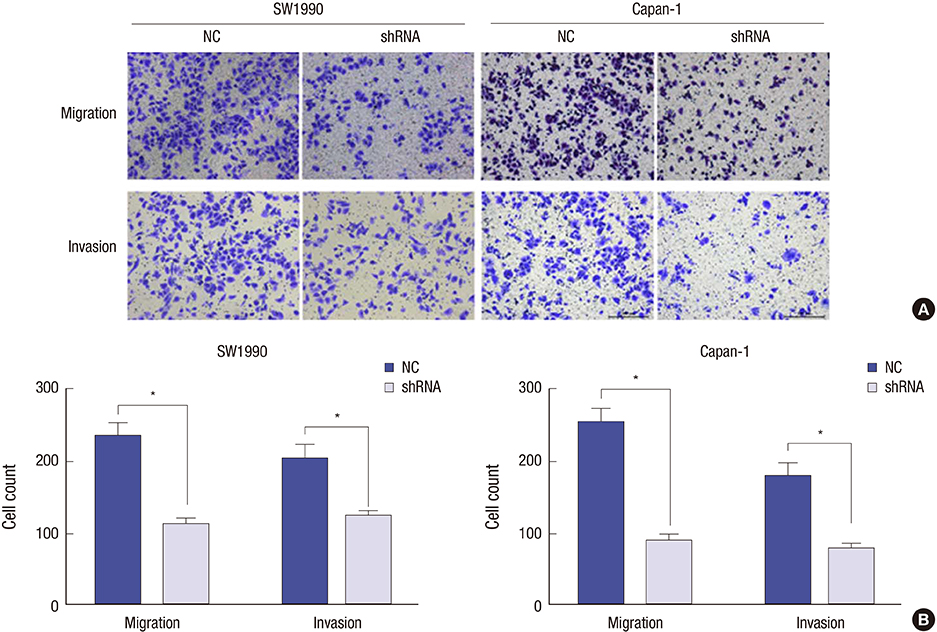
- Identifying a target molecule that is crucially involved in pancreatic tumor growth and metastasis is necessary in developing an effective treatment. The study aimed to investigate the role of the eukaryotic translation initiation factor 3a (eIF3a) in the cell proliferation and motility in pancreatic cancer. Our data showed that the expression of eIF3a was upregulated in pancreatic ductal adenocarcinoma as compared with its expression in normal pancreatic tissues. Knockdown of eIF3a by a specific shRNA caused significant decreases in cell proliferation and clonogenic abilities in pancreatic cancer SW1990 and Capan-1 cells. Consistently, the pancreatic cancer cell growth rates were also impaired in xenotransplanted mice. Moreover, wound-healing assay showed that depletion of eIF3a significantly slowed down the wound recovery processes in SW1990 and Capan-1 cells. Transwell migration and invasion assays further showed that cell migration and invasion abilities were significantly inhibited by knockdown of eIF3a in SW1990 and Capan-1 cells. Statistical analysis of eIF3a expression in 140 cases of pancreatic ductal adenocarcinoma samples revealed that eIF3a expression was significantly associated with tumor metastasis and TNM staging. These analyses suggest that eIF3a contributes to cell proliferation and motility in pancreatic ductal adenocarcinoma.
MeSH Terms
-
Aged
Animals
Carcinoma, Pancreatic Ductal/metabolism/*pathology/therapy
Cell Line, Tumor
Cell Movement
Cell Proliferation
Eukaryotic Initiation Factor-3/antagonists & inhibitors/genetics/*metabolism
Female
Humans
Immunohistochemistry
Male
Mice
Mice, Inbred BALB C
Mice, Nude
Middle Aged
Pancreatic Neoplasms/metabolism/*pathology/therapy
RNA Interference
RNA, Small Interfering/administration & dosage/metabolism
Transplantation, Heterologous
Eukaryotic Initiation Factor-3
RNA, Small Interfering
Figure
Reference
-
1. Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004; 363:1049–1057.2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65:5–29.3. Van Cutsem E, Aerts R, Haustermans K, Topal B, Van Steenbergen W, Verslype C. Systemic treatment of pancreatic cancer. Eur J Gastroenterol Hepatol. 2004; 16:265–274.4. Li YY, Popivanova BK, Nagai Y, Ishikura H, Fujii C, Mukaida N. Pim-3, a proto-oncogene with serine/threonine kinase activity, is aberrantly expressed in human pancreatic cancer and phosphorylates bad to block bad-mediated apoptosis in human pancreatic cancer cell lines. Cancer Res. 2006; 66:6741–6747.5. Maitra U, Stringer EA, Chaudhuri A. Initiation factors in protein biosynthesis . Annu Rev Biochem. 1982; 51:869–900.6. Dong Z, Zhang JT. Initiation factor eIF3 and regulation of mRNA translation, cell growth, and cancer. Crit Rev Oncol Hematol. 2006; 59:169–180.7. Yin JY, Dong Z, Liu ZQ, Zhang JT. Translational control gone awry: a new mechanism of tumorigenesis and novel targets of cancer treatments. Biosci Rep. 2011; 31:1–15.8. Unbehaun A, Borukhov SI, Hellen CU, Pestova TV. Release of initiation factors from 48S complexes during ribosomal subunit joining and the link between establishment of codon-anticodon base-pairing and hydrolysis of eIF2-bound GTP. Genes Dev. 2004; 18:3078–3093.9. Asano K, Vornlocher HP, Richter-Cook NJ, Merrick WC, Hinnebusch AG, Hershey JW. Structure of cDNAs encoding human eukaryotic initiation factor 3 subunits. Possible roles in RNA binding and macromolecular assembly. J Biol Chem. 1997; 272:27042–27052.10. Zhou C, Arslan F, Wee S, Krishnan S, Ivanov AR, Oliva A, Leatherwood J, Wolf DA. PCI proteins eIF3e and eIF3m define distinct translation initiation factor 3 complexes. BMC Biol. 2005; 3:14.11. Saletta F, Suryo Rahmanto Y, Richardson DR. The translational regulator eIF3a: the tricky eIF3 subunit. Biochim Biophys Acta. 2010; 1806:275–286.12. Zhang L, Pan X, Hershey JW. Individual overexpression of five subunits of human translation initiation factor eIF3 promotes malignant transformation of immortal fibroblast cells. J Biol Chem. 2007; 282:5790–5800.13. Dong Z, Liu Y, Zhang JT. Regulation of ribonucleotide reductase M2 expression by the upstream AUGs. Nucleic Acids Res. 2005; 33:2715–2725.14. Smith MD, Gu Y, Querol-Audí J, Vogan JM, Nitido A, Cate JH. Human-like eukaryotic translation initiation factor 3 from Neurospora crassa. PLoS One. 2013; 8:e78715.15. Aylett CH, Boehringer D, Erzberger JP, Schaefer T, Ban N. Structure of a yeast 40S-eIF1-eIF1A-eIF3-eIF3j initiation complex. Nat Struct Mol Biol. 2015; 22:269–271.16. LeFebvre AK, Korneeva NL, Trutschl M, Cvek U, Duzan RD, Bradley CA, Hershey JW, Rhoads RE. Translation initiation factor eIF4G-1 binds to eIF3 through the eIF3e subunit. J Biol Chem. 2006; 281:22917–22932.17. Dong Z, Arnold RJ, Yang Y, Park MH, Hrncirova P, Mechref Y, Novotny MV, Zhang JT. Modulation of differentiation-related gene 1 expression by cell cycle blocker mimosine, revealed by proteomic analysis. Mol Cell Proteomics. 2005; 4:993–1001.18. He J, Shi J, Fu X, Mao L, Zhou T, Qiu Y, Zhu B. The clinicopathologic and prognostic significance of gross classification on solitary hepatocellular carcinoma after hepatectomy. Medicine (Baltimore). 2015; 94:e1331.19. Shen J, Yin JY, Li XP, Liu ZQ, Wang Y, Chen J, Qu J, Xu XJ, McLeod HL, He YJ, et al. The prognostic value of altered eIF3a and its association with p27 in non-small cell lung cancers. PLoS One. 2014; 9:e96008.20. Chen G, Burger MM. p150 overexpression in gastric carcinoma: the association with p53, apoptosis and cell proliferation. Int J Cancer. 2004; 112:393–398.21. Chen G, Burger MM. p150 expression and its prognostic value in squamous-cell carcinoma of the esophagus. Int J Cancer. 1999; 84:95–100.22. Haybaeck J, O’Connor T, Spilka R, Spizzo G, Ensinger C, Mikuz G, Brunhuber T, Vogetseder A, Theurl I, Salvenmoser W, et al. Overexpression of p150, a part of the large subunit of the eukaryotic translation initiation factor 3, in colon cancer. Anticancer Res. 2010; 30:1047–1055.23. Spilka R, Laimer K, Bachmann F, Spizzo G, Vogetseder A, Wieser M, Müller H, Haybaeck J, Obrist P. Overexpression of eIF3a in squamous cell carcinoma of the oral cavity and its putative relation to chemotherapy response. J Oncol. 2012; 2012:901956.24. Bachmann F, Bänziger R, Burger MM. Cloning of a novel protein overexpressed in human mammary carcinoma. Cancer Res. 1997; 57:988–994.25. Wu YH, Li XW, Li WQ, Li XH, Li YJ, Hu GY, Liu ZQ, Li D. Fluorofenidone attenuates bleomycin-induced pulmonary fibrosis by inhibiting eukaryotic translation initiation factor 3a (eIF3a) in rats. Eur J Pharmacol. 2016; 773:42–50.26. Yin JY, Shen J, Dong ZZ, Huang Q, Zhong MZ, Feng DY, Zhou HH, Zhang JT, Liu ZQ. Effect of eIF3a on response of lung cancer patients to platinum-based chemotherapy by regulating DNA repair. Clin Cancer Res. 2011; 17:4600–4609.27. Liu K, Lei Z, Yao H, Lei S, Zhao H. Impact of a Eukaryotic Translation Initiation Factor 3a Polymorphism on Susceptibility to Gastric Cancer. Med Princ Pract. 2016; Forthcoming.28. Spilka R, Ernst C, Bergler H, Rainer J, Flechsig S, Vogetseder A, Lederer E, Benesch M, Brunner A, Geley S, et al. eIF3a is over-expressed in urinary bladder cancer and influences its phenotype independent of translation initiation. Cell Oncol (Dordr). 2014; 37:253–267.29. Liu Z, Dong Z, Yang Z, Chen Q, Pan Y, Yang Y, Cui P, Zhang X, Zhang JT. Role of eIF3a (eIF3 p170) in intestinal cell differentiation and its association with early development. Differentiation. 2007; 75:652–661.30. Zhang Y, Yu JJ, Tian Y, Li ZZ, Zhang CY, Zhang SF, Cao LQ, Zhang Y, Qian CY, Zhang W, et al. eIF3a improve cisplatin sensitivity in ovarian cancer by regulating XPC and p27Kip1 translation. Oncotarget. 2015; 6:25441–25451.31. Olson JE, Wang X, Goode EL, Pankratz VS, Fredericksen ZS, Vierkant RA, Pharoah PD, Cerhan JR, Couch FJ. Variation in genes required for normal mitosis and risk of breast cancer. Breast Cancer Res Treat. 2010; 119:423–430.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Translational Regulation: A Novel Target for Breast Cancer Therapy
- Neuronal activation increases the density of eukaryotic translation initiation factor 4E mRNA clusters in dendrites of cultured hippocampal neurons
- Human Leptin Protein Induces Proliferation of A549 Cells via Inhibition of PKR-Like ER Kinase and Activating Transcription Factor-6 Mediated Apoptosis
- Suppression of Antimicrobial Defense and Stabilization of STAT3 by IRAK-M Expression in Tumor Cells Promotes Colorectal Carcinogenesis
- Inhibition of Mast Cell Function and Proliferation by mTOR Activator MHY1485