J Bacteriol Virol.
2019 Sep;49(3):124-132. 10.4167/jbv.2019.49.3.124.
Recharacterization of the Canine Adenovirus Type 1 Vaccine Strain based on the Biological and Molecular Properties
- Affiliations
-
- 1Viral Disease Research Division, Animal and Plant Quarantine Agency, MAFRA, Gimcheon, 39660, Republic of Korea. yangdk@korea.kr
- 2KBNP Technology Institute, KBNP, Yesan-gun, 32417, Republic of Korea.
- KMID: 2468032
- DOI: http://doi.org/10.4167/jbv.2019.49.3.124
Abstract
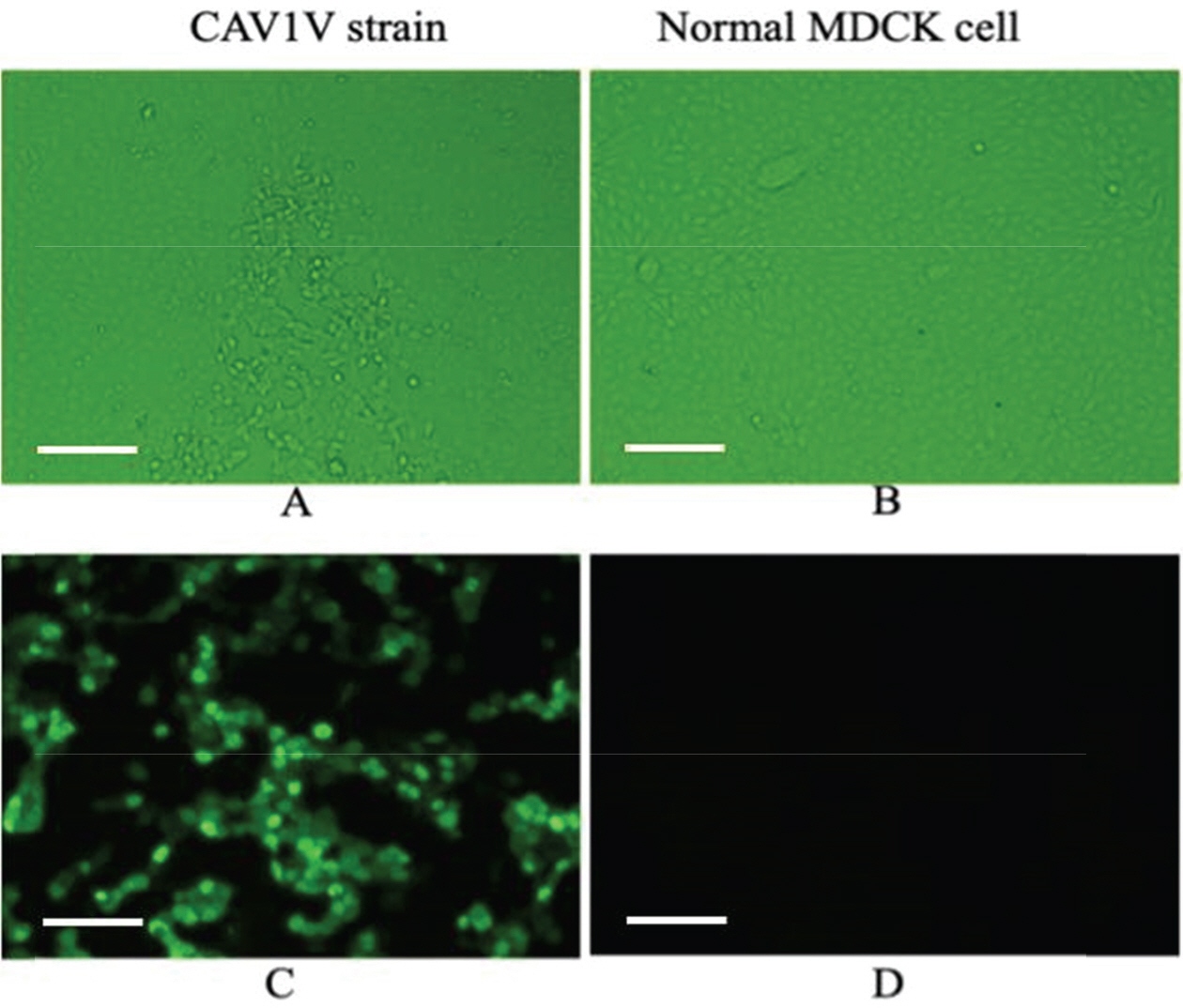
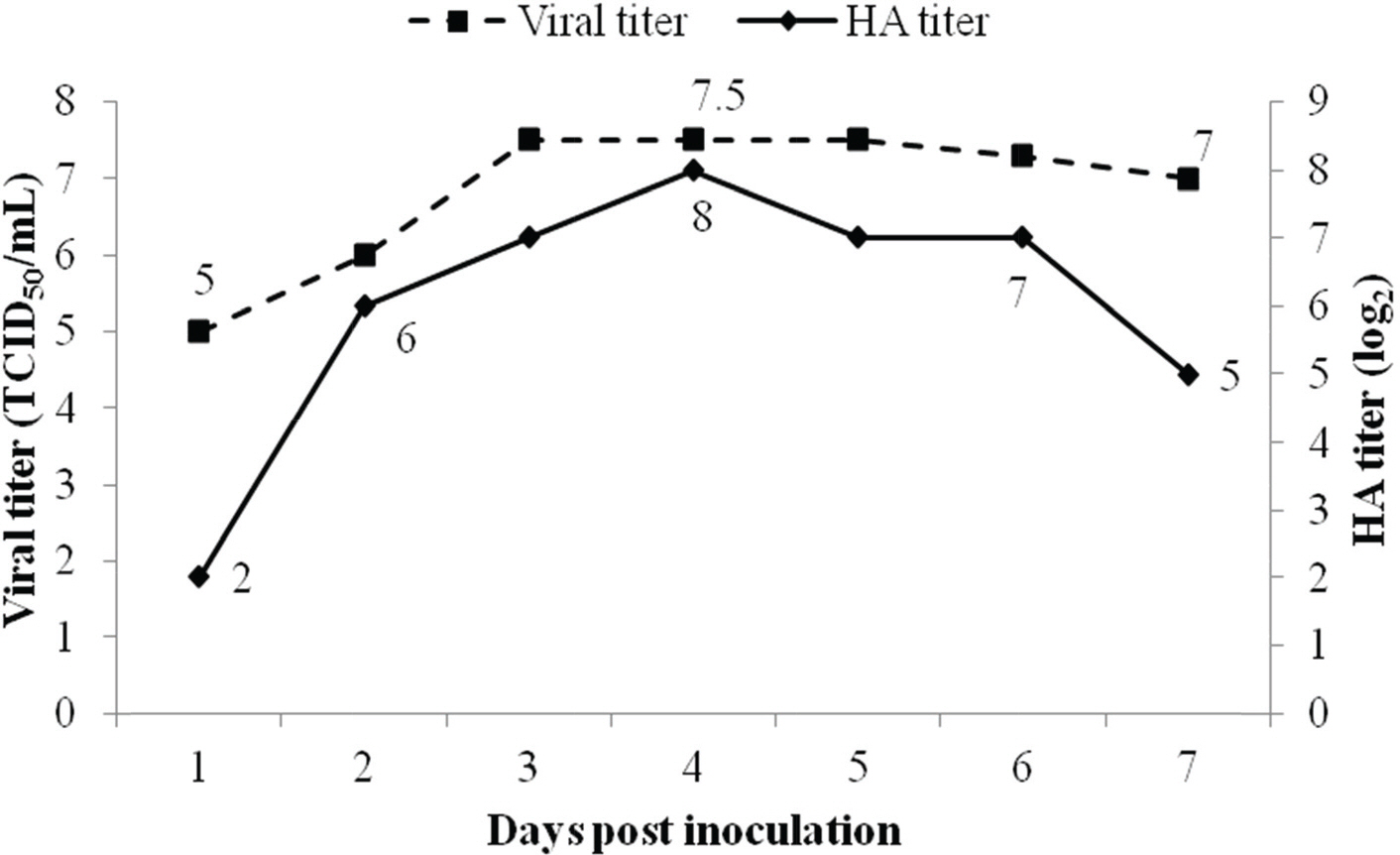
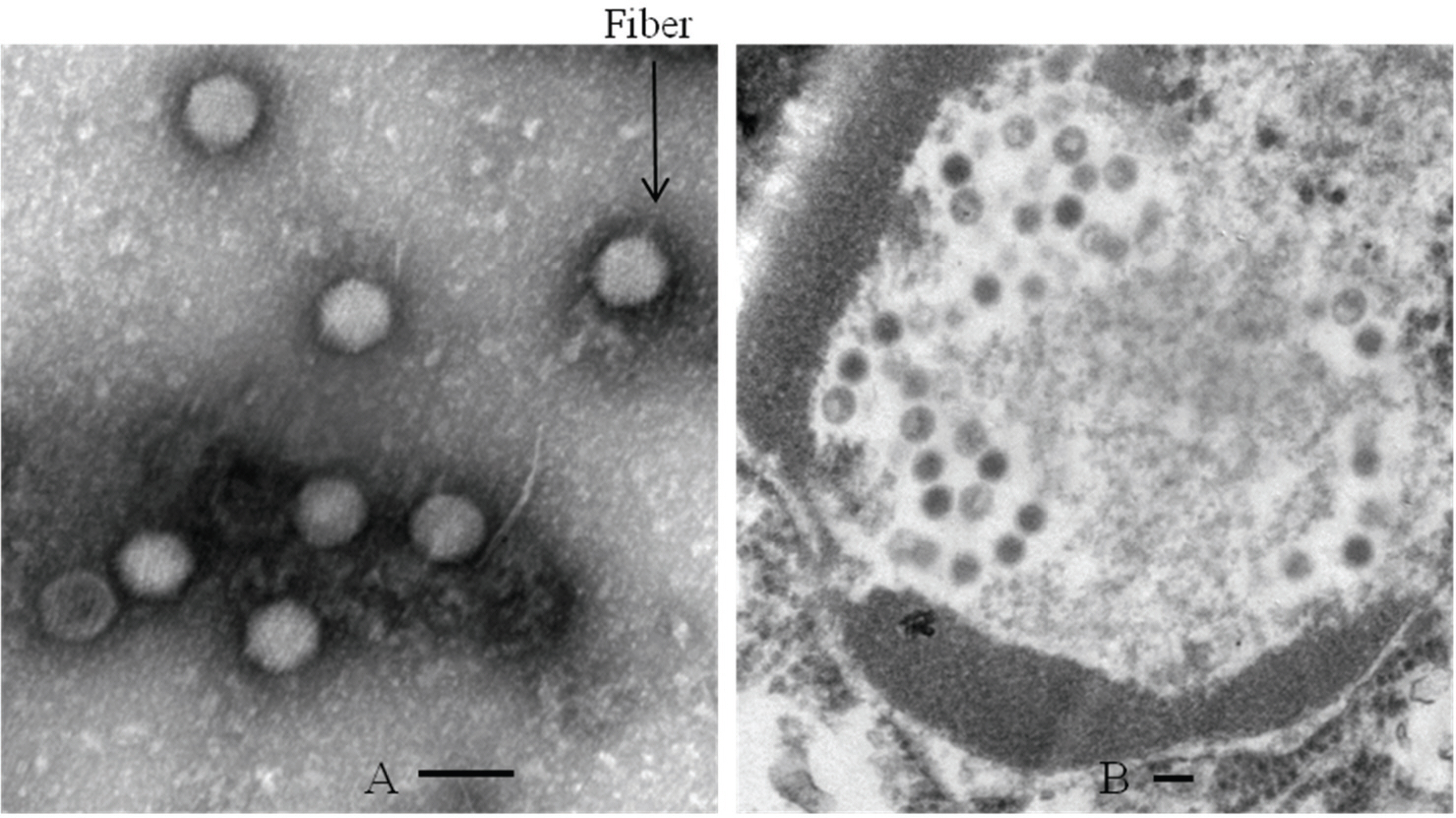
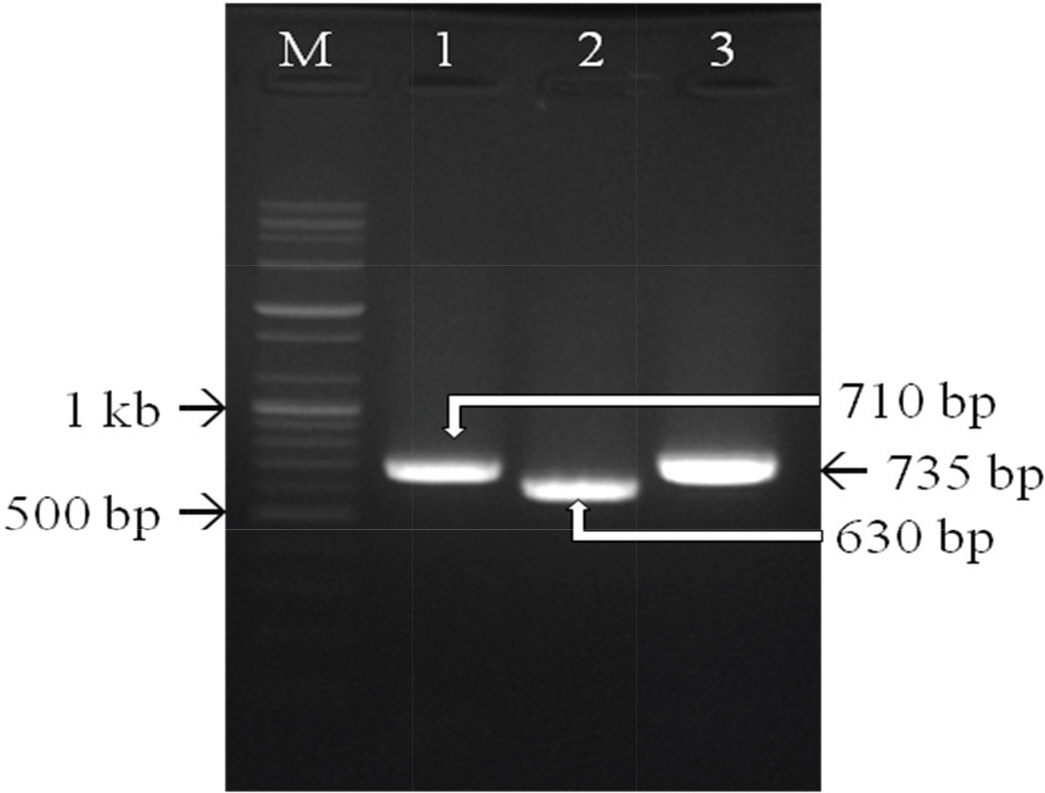
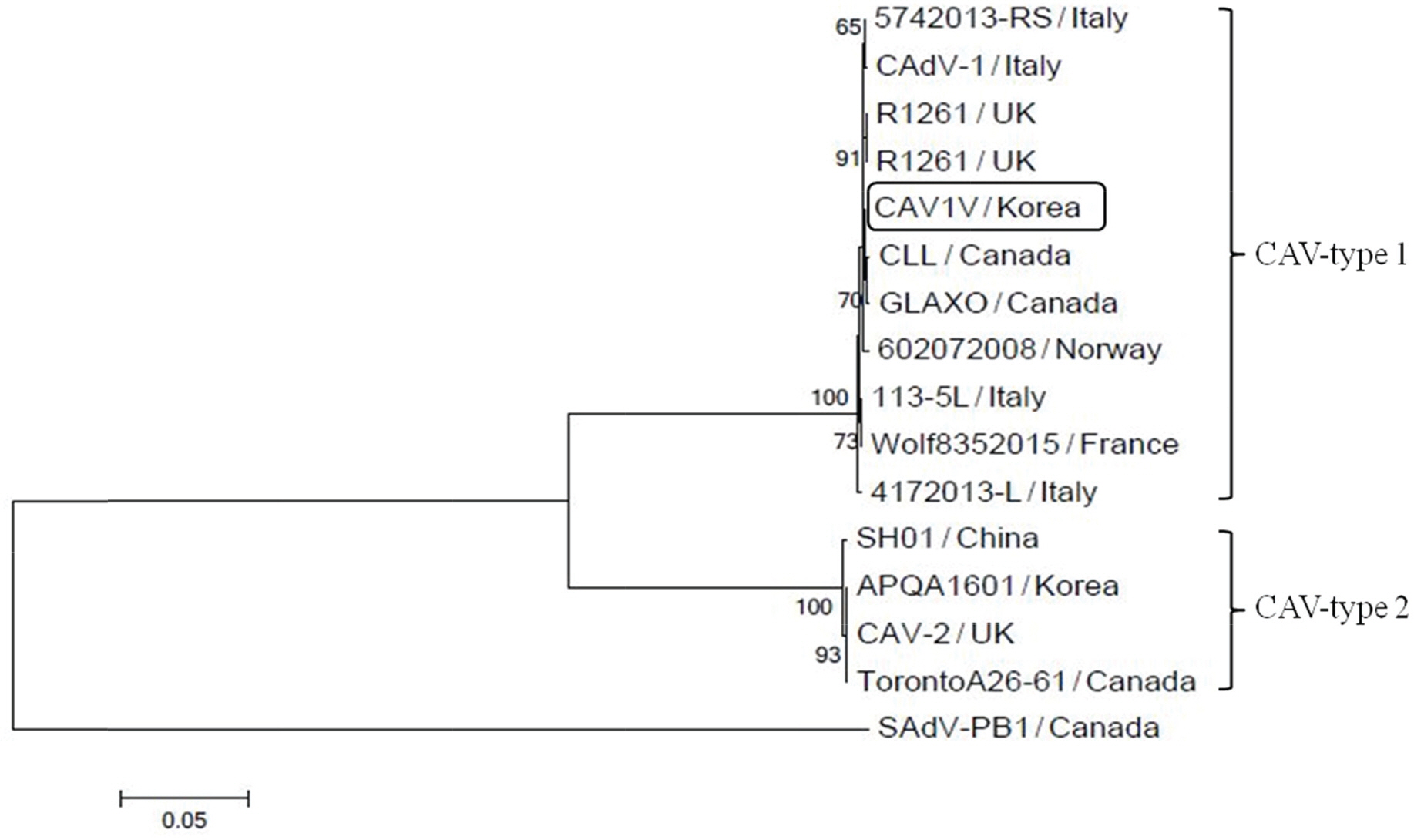
- Canine adenovirus type 1 (CAV-1) infection results in hepatitis in dogs. In this study, we investigated the biologic and genetic characteristics of the CAV-1 vaccine strain (CAV1V) to improve quality control about CAV vaccine. The identity of CAV1V as CAV-1 was confirmed based on its cytopathic effects and the results of hemagglutination (HA) and immunofluorescence assays, and electron microscopy. The CAV1V strain reached 10(7.5) TCID(50)/mL in MDCK cells at 4 days post-inoculation and exhibited hemmagglutination activity of 256 U using guinea pig erythrocytes. Intranuclear fluorescence in the infected cells was observed and typical adenoviruses were observed in electon microscope. CAV1V strain was identified as a CAV-1 strain by nucleotide sequence analysis. In a comparison of the nucleotide sequences of the fiber genes of several CAV strains, CAV1V showed the highest similarity (99.8%) with the GLAXO strain, which was isolated in Canada. Our biological characterization of CAV1V will facilitate quality control of the canine hepatitis vaccine.
MeSH Terms
Figure
Cited by 1 articles
-
Immunogenicity of a new, inactivated canine adenovirus type 2 vaccine for dogs
Dong-Kun Yang, Ha-Hyun Kim, Jae Young Yoo, Miryeon Ji, Bok Hee Han, Subin Oh, Bang-Hun Hyun
Clin Exp Vaccine Res. 2020;9(1):40-47. doi: 10.7774/cevr.2020.9.1.40.
Reference
-
1). Hu RL, Huang G, Qiu W, Zhong ZH, Xia XZ, Yin Z. Detection and differentiation of CAV-1 and CAV-2 by polymerase chain reaction. Vet Res Commun. 2001; 25:77–84.2). Linné T. Differences in the E3 regions of the canine adenovirus type 1 and type 2. Virus Res. 1992; 23:119–33.
Article3). Walker D, Abbondati E, Cox AL, Mitchell GB, Pizzi R, Sharp CP, et al. Infectious canine hepatitis in red foxes (Vulpes vulpes) in wildlife rescue centres in the UK. Vet Rec. 2016; 178:421.
Article4). Yang DK, Kim HH, Yoon SS, Lee H, Cho IS. Isolation and identification of canine adenovirus type 2 from a naturally infected dog in Korea. Korean J Vet Res. 2018; 58:177–82.
Article5). Aoki E, Soma T, Yokoyama M, Matsubayashi M, Sasai K. Surveillance for Antibodies against Six Canine Viruses in Wild Raccoons (Procyon Lotor) in Japan. J Wildl Dis. 2017; 53:761–8.
Article6). Yang DK, Kim HH, Yoon SS, Ji M, Cho IS. Incidence and Sero-survey of Canine Adenovirus Type 2 in Various Animal Species. J Bacteriol Virol. 2018; 48:102–8.
Article7). Loustalot F, Kremer EJ, Salinas S. The Intracellular Domain of the Coxsackievirus and Adenovirus Receptor Differentially Influences Adenovirus Entry. J Virol. 2015; 89:9417–26.
Article8). Tham KM, Horner GW, Hunter R. Isolation and identification of canine adenovirus type-2 from the upper respiratory tract of a dog. N Z Vet J. 1998; 46:102–5.
Article9). Shenk TE. Adenoviridae: The Viruses and Their Replication. Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 4th ed. 2. Philadelphia: Lippincott Williams & Wilkins;2001. p. 2265–300.10). Walker D, Fee SA, Hartley G, Learmount J, O'Hagan MJ, Meredith AL, et al. Serological and molecular epidemiology of canine adenovirus type 1 in red foxes (Vulpes vulpes) in the United Kingdom. Sci Rep. 2016; 6:36051.
Article11). Whetstone CA. Monoclonal antibodies to canine adenoviruses 1 and 2 that are type-specific by virus neutralization and immunofluorescence. Vet Microbiol. 1988; 16:1–8.
Article12). Morrison MD, Onions DE, Nicolson L. Complete DNA sequence of canine adenovirus type 1. J Gen Virol. 1997; 78:873–8.
Article13). Pizzurro F, Marcacci M, Zaccaria G, Orsini M, Cito F, Rosamilia A, et al. Genome Sequence of Canine Adenovirus Type 1 Isolated from a Wolf (Canis lupus) in Southern Italy. Genome Announc. 2017; 5.
Article14). Green NM, Wrigley NG, Russell WC, Martin SR, McLachlan AD. Evidence for a repeating cross-beta sheet structure in the adenovirus fibre. EMBO J. 1983; 2:1357–65.
Article15). Mei YF, Wadell G. Hemagglutination properties and nucleotide sequence analysis of the fiber gene of adenovirus genome types 11p and 11a. Virology. 1993; 194:453–62.
Article16). Reed LJ, Muench H. A Simple Method of Estimating Fifty Per Cent Endpoints. Am J Epidemiology. 1938; 27:493–7.17). Choi JW, Lee HK, Kim SH, Kim YH, Lee KK, Lee MH, et al. Canine adenovirus type 1 in a fennec fox (Vulpes zerda). J Zoo Wildl Med. 2014; 45:947–50.
Article18). Wong M, Woolford L, Hasan NH, Hemmatzadeh F. A Novel Recombinant Canine Adenovirus Type 1 Detected from Acute Lethal Cases of Infectious Canine Hepatitis. Viral Immunol. 2017; 30:258–63.
Article19). Takamura K, Ajiki M, Hiramatsu K, Takemitsu S, Nakai M, Sasaki N. Isolation and properties of adenovirus from canine respiratory tract. Nihon Juigaku Zasshi. 1982; 44:355–7.
Article20). Kennedy FA, Mullaney TP. Disseminated adenovirus infection in a cat. J Vet Diagn Invest. 1993; 5:273–6.
Article21). Chaturvedi U, Tiwari AK, Ratta B, Ravindra PV, Rajawat YS, Palia SK, et al. Detection of canine adenoviral infections in urine and faeces by the polymerase chain reaction. J Virol Methods. 2008; 149:260–3.
Article22). Sanjuá n R, Domingo-Calap P. Mechanisms of viral mutation. Cell Mol Life Sci. 2016; 73:4433–48.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity of a new, inactivated canine adenovirus type 2 vaccine for dogs
- Isolation and identification of canine adenovirus type 2 from a naturally infected dog in Korea
- Adenovirus Vectors: Excellent Tools for Vaccine Development
- Development of a novel vaccine against canine parvovirus infection with a clinical isolate of the type 2b strain
- Establishment of multiplex RT-PCR for differentiation between rabies virus with and that without mutation at position 333 of glycoprotein