J Clin Neurol.
2020 Jan;16(1):37-45. 10.3988/jcn.2020.16.1.37.
A Cortical Substrate for Square-Wave Jerks in Progressive Supranuclear Palsy
- Affiliations
-
- 1Department of Neurology, Eginition Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece. eanagnost@eginitio.uoa.gr
- 22nd Department of Radiology, University General Hospital ‘Attikon,’ School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
- KMID: 2467782
- DOI: http://doi.org/10.3988/jcn.2020.16.1.37
Abstract
- BACKGROUND AND PURPOSE
Square-wave jerks (SWJs) are the most common saccadic intrusion in progressive supranuclear palsy (PSP), but their genesis is uncertain. We aimed to determine the characteristics of SWJs in PSP (the Richardson subtype) and Parkinson's disease (PD) and to map the brain structures responsible for abnormal SWJ parameters in PSP.
METHODS
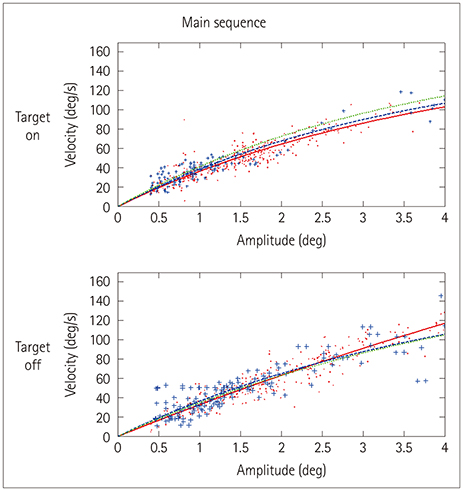
Eye movements in 12 patients with PSP, 12 patients with PD, and 12 age-matched healthy controls were recorded using an infrared corneal reflection device. The rate, mean amplitude, and velocity of SWJs were analyzed offline. Voxel-based morphometry using a 3-Tesla MRI scanner was performed to relate changes in brain volume to SWJ parameters.
RESULTS
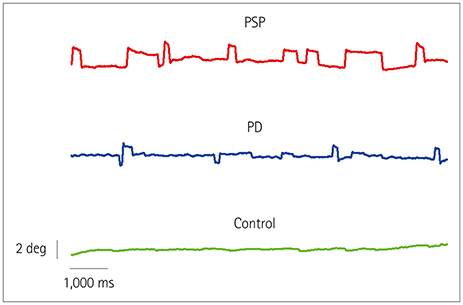
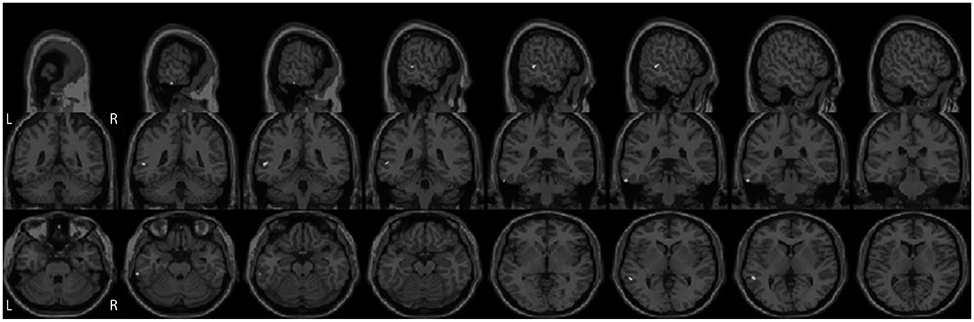
The SWJ rate was more than threefold higher in PSP patients than in both PD patients and controls (mean rates: 33.5, 10.3, and 4.3 SWJs per minute, respectively). The volumes of neither the midbrain nor other infratentorial brain regions were correlated with the SWJ rate. Instead, highly significant associations were found for atrophy in the superior, middle, and inferior temporal gyri in the PSP group.
CONCLUSIONS
SWJs in PSP are not mediated by midbrain atrophy. Instead, supratentorial cortical structures located mainly in the temporal lobe appear to be deeply involved in the generation of abnormally high SWJ rates in these patients. Known anatomical connections of the temporal lobe to the superior colliculus and the cerebellum might play a role in SWJ genesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Troost BT, Daroff RB. The ocular motor defects in progressive supranuclear palsy. Ann Neurol. 1977; 2:397–403.
Article2. Spieker S, Schulz JB, Petersen D, Fetter M, Klockgether T, Dichgans J. Fixation instability and oculomotor abnormalities in Friedreich's ataxia. J Neurol. 1995; 242:517–521.
Article3. Ribaï P, Pousset F, Tanguy ML, Rivaud-Pechoux S, Le Ber I, Gasparini F, et al. Neurological, cardiological, and oculomotor progression in 104 patients with Friedreich ataxia during long-term follow-up. Arch Neurol. 2007; 64:558–564.
Article4. Fahey MC, Cremer PD, Aw ST, Millist L, Todd MJ, White OB, et al. Vestibular, saccadic and fixation abnormalities in genetically confirmed Friedreich ataxia. Brain. 2008; 131:1035–1045.
Article5. Verhagen WI, Huygen PL, Arts WF. Multi-system signs and symptoms in X-linked ataxia carriers. J Neurol Sci. 1996; 140:85–90.
Article6. Bürk K, Fetter M, Abele M, Laccone F, Brice A, Dichgans J, et al. Autosomal dominant cerebellar ataxia type I: oculomotor abnormalities in families with SCA1, SCA2, and SCA3. J Neurol. 1999; 246:789–797.
Article7. Christova P, Anderson JH, Gomez CM. Impaired eye movements in presymptomatic spinocerebellar ataxia type 6. Arch Neurol. 2008; 65:530–536.
Article8. Clausi S, De Luca M, Chiricozzi FR, Tedesco AM, Casali C, Molinari M, et al. Oculomotor deficits affect neuropsychological performance in oculomotor apraxia type 2. Cortex. 2013; 49:691–701.
Article9. Passo M, Shults WT, Talbot T, Palmer EA. Acquired esotropia. A manifestation of Chiari I malformation. J Clin Neuroophthalmol. 1984; 4:151–154.10. Anagnostou E, Papageorgiou SG, Potagas C, Alexakis T, Kalfakis N, Anastasopoulos D. Square-wave jerks and smooth pursuit impairment as subtle early signs of brain involvement in Langerhans' cell histiocytosis. Clin Neurol Neurosurg. 2008; 110:286–290.
Article11. Brokalaki C, Kararizou E, Dimitrakopoulos A, Evdokimidis I, Anagnostou E. Square-wave ocular oscillation and ataxia in an anti-GAD-positive individual with hypothyroidism. J Neuroophthalmol. 2015; 35:390–395.
Article12. Dell'Osso LF, Troost BT, Daroff RB. Macro square wave jerks. Neurology. 1975; 25:975–979.13. Otero-Millan J, Macknik SL, Serra A, Leigh RJ, Martinez-Conde S. Triggering mechanisms in microsaccade and saccade generation: a novel proposal. Ann N Y Acad Sci. 2011; 1233:107–116.
Article14. Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990; 302:330–348.
Article15. Scudder CA, McGee DM, Balaban CD. Connections of monkey saccade-related fastigial nucleus neurons revealed by anatomical and physiological methods. Soc Neurosci Abstr. 2000; 26:971.16. Williams DR, De Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson's syndrome and PSP-parkinsonism. Brain. 2005; 128:1247–1258.
Article17. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992; 55:181–184.
Article18. Leigh RJ, Zee DS. The neurology of eye movements. 4th ed. New York: Oxford University Press;2006.19. Anagnostou E, Kemanetzoglou E, Papadimas G, Kararizou E, Evdokimidis I. Extraocular muscle function in adult-onset Pompe disease tested by saccadic eye movements. Neuromuscul Disord. 2014; 24:1073–1078.
Article20. Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math Biosci. 1975; 24:191–204.
Article21. Baloh RW, Sills AW, Kumley WE, Honrubia V. Quantitative measurement of saccade amplitude, duration, and velocity. Neurology. 1975; 25:1065–1070.
Article22. Versino M, Rossi B, Beltrami G, Sandrini G, Cosi V. Ocular motor myotonic phenomenon in myotonic dystrophy. J Neurol Neurosurg Psychiatry. 2002; 72:236–240.
Article23. Stonnington CM, Tan G, Klöppel S, Chu C, Draganski B, Jack CR Jr, et al. Interpreting scan data acquired from multiple scanners: a study with Alzheimer's disease. Neuroimage. 2008; 39:1180–1185.
Article24. Kostić VS, Agosta F, Petrović I, Galantucci S, Spica V, Jecmenica-Lukic M, et al. Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology. 2010; 75:857–863.
Article25. Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010; 53:1244–1255.
Article26. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003; 19:1233–1239.
Article27. Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004; 21:450–455.
Article28. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002; 15:273–289.
Article29. Highstein S, Cohen B, Mones R. Changes in saccadic eye movements of patients with Parkinson's disease before and after L-dopa. Trans Am Neurol Assoc. 1969; 94:277–279.30. Dix MR, Harrison MJ, Lewis PD. Progressive supranuclear palsy (the Steele-Richardson-Olszewski syndrome). A report of 9 cases with particular reference to the mechanism of the oculomotor disorder. J Neurol Sci. 1971; 13:237–256.31. Anderson T, Luxon L, Quinn N, Daniel S, David Marsden C, Bronstein A. Oculomotor function in multiple system atrophy: clinical and laboratory features in 30 patients. Mov Disord. 2008; 23:977–984.
Article32. Gorges M, Müller HP, Lulé D, Ludolph AC, Pinkhardt EH, Kassubek J. Functional connectivity within the default mode network is associated with saccadic accuracy in Parkinson’s disease: a resting-state FMRI and videooculographic study. Brain Connect. 2013; 3:265–272.
Article33. Rosskopf J, Gorges M, Müller HP, Lulé D, Uttner I, Ludolph AC, et al. Intrinsic functional connectivity alterations in progressive supranuclear palsy: differential effects in frontal cortex, motor, and midbrain networks. Mov Disord. 2017; 32:1006–1015.
Article34. Vintonyak O, Gorges M, Müller HP, Pinkhardt EH, Ludolph AC, Huppertz HJ, et al. Patterns of eye movement impairment correlate with regional brain atrophy in neurodegenerative parkinsonism. Neurodegener Dis. 2017; 17:117–126.
Article35. Wakabayashi K, Takahashi H. Pathological heterogeneity in progressive supranuclear palsy and corticobasal degeneration. Neuropathology. 2004; 24:79–86.
Article36. Jellinger KA. Neuropathology and pathogenesis of extrapyramidal movement disorders: a critical update-I. Hypokinetic-rigid movement disorders. J Neural Transm (Vienna). 2019; 126:933–995.
Article37. Josephs KA, Xia R, Mandrekar J, Gunter JL, Senjem ML, Jack CR Jr, et al. Modeling trajectories of regional volume loss in progressive supranuclear palsy. Mov Disord. 2013; 28:1117–1124.
Article38. Caso F, Agosta F, Volonté MA, Ferraro PM, Tiraboschi P, Copetti M, et al. Cognitive impairment in progressive supranuclear palsy-Richardson's syndrome is related to white matter damage. Parkinsonism Relat Disord. 2016; 31:65–71.
Article39. Höglinger GU, Schöpe J, Stamelou M, Kassubek J, Del Ser T, Boxer AL, et al. Longitudinal magnetic resonance imaging in progressive supranuclear palsy: a new combined score for clinical trials. Mov Disord. 2017; 32:842–852.
Article40. Agosta F, Caso F, Ječmenica-Lukić M, Petrović IN, Valsasina P, Meani A, et al. Tracking brain damage in progressive supranuclear palsy: a longitudinal MRI study. J Neurol Neurosurg Psychiatry. 2018; 89:696–701.
Article41. Jones A, Friedland RP, Koss B, Stark L, Thompkins-Ober BA. Saccadic intrusions in Alzheimer-type dementia. J Neurol. 1983; 229:189–194.
Article42. Parkinson J, Maxner C. Eye movement abnormalities in Alzheimer disease: case presentation and literature review. Am Orthopt J. 2005; 55:90–96.
Article43. Shakespeare TJ, Kaski D, Yong KX, Paterson RW, Slattery CF, Ryan NS, et al. Abnormalities of fixation, saccade and pursuit in posterior cortical atrophy. Brain. 2015; 138:1976–1991.
Article44. Sharpe JA, Herishanu YO, White OB. Cerebral square wave jerks. Neurology. 1982; 32:57–62.
Article45. Cranford JL, Ladner SJ, Campbell CB, Neff WD. Efferent projections of the insular and temporal neocortex of the cat. Brain Res. 1976; 117:195–210.
Article46. Maioli MG, Domeniconi R, Squatrito S, Riva Sanseverino E. Projections from cortical visual areas of the superior temporal sulcus to the superior colliculus, in macaque monkeys. Arch Ital Biol. 1992; 130:157–166.47. Webster MJ, Bachevalier J, Ungerleider LG. Transient subcortical connections of inferior temporal areas TE and TEO in infant macaque monkeys. J Comp Neurol. 1995; 352:213–226.
Article48. Ruwaldt MM, Snider RS. Projections of vestibular areas of cerebellum to the cerebrum. J Comp Neurol. 1956; 104:387–401.
Article49. Brodal P. The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain. 1978; 101:251–283.
Article50. Schmahmann JD, Pandya DN. Projections to the basis pontis from the superior temporal sulcus and superior temporal region in the rhesus monkey. J Comp Neurol. 1991; 308:224–248.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Progressive Supranuclear Palsy Presenting as Primary Progressive Aphasia
- Cretzfeldt-Jakob Disease Mimicking Progressive Supranuclear Palsy Presenting With Supranuclear Gaze Palsy and Early Falling
- A Case of Progressive Supranuclear Palsy with Schizophrenic Symptoms
- Probable Creutzfeldt-Jakob Disease Presenting as Progressive Supranuclear Palsy
- A Case of Bilateral Vocal Cord Paralysis from Progressive Supranuclear Palsy