J Clin Neurol.
2019 Oct;15(4):555-563. 10.3988/jcn.2019.15.4.555.
Association Analysis of Interleukin-1β, Interleukin-6, and HMGB1 Variants with Postictal Serum Cytokine Levels in Children with Febrile Seizure and Generalized Epilepsy with Febrile Seizure Plus
- Affiliations
-
- 1Department of Pediatrics, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea. jechoi66@snu.ac.kr
- 2Department of Pediatrics, Dankook University Hospital, Cheonan, Korea.
- 3Department of Pediatrics, Pediatric Clinical Neuroscience Center, Seoul National University Children's Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Pediatrics, Seoul National University Bundang Hospital, Seoul, Korea.
- 5Department of Biostatistics, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Microbiology, Brain Korea 21 Plus Project for Medical Science, Severance Biomedical Science Institute and Institute for Immunology and Immunological Diseases, Yonsei University College of Medicine, Seoul, Korea. jsshin6203@yuhs.ac
- KMID: 2467766
- DOI: http://doi.org/10.3988/jcn.2019.15.4.555
Abstract
- BACKGROUND AND PURPOSE
Febrile seizure (FS) is a unique type of seizure that only occurs during childhood. Genelized epilepsy with febrile seizure plus (GEFS+) is a familial epilepsy syndrome associated with FS and afebrile seizure (AFS). Both seizure types are related to fever, but whether genetic susceptibility to inflammation is implicated in them is still unclear. To analyze the associations between postictal serum cytokine levels and genetic variants in the cytokine genes interleukin (IL)-1β, IL-6, and high mobility group box-1 (HMGB1) in FS and GEFS+.
METHODS
Genotyping was performed in 208 subjects (57 patients with FS, 43 patients with GEFS+, and 108 controls) with the SNaPshot assay for IL-1β-31 (rs1143627), IL-1β-511 (rs16944), IL-6-572 (rs1800796), and HMGB1 3814 (rs2249825). Serum IL-1β, IL-6, and HMGB1 levels were analyzed within 2 hours after seizure attacks using the ELISA in only 68 patients (38 FS, 10 GEFS+, and 20 controls). The allele distribution, genotype distribution, and correlations with serum cytokine levels were analyzed.
RESULTS
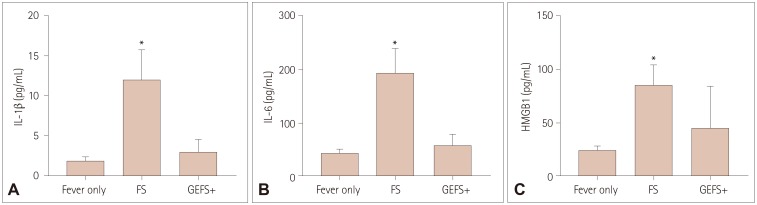
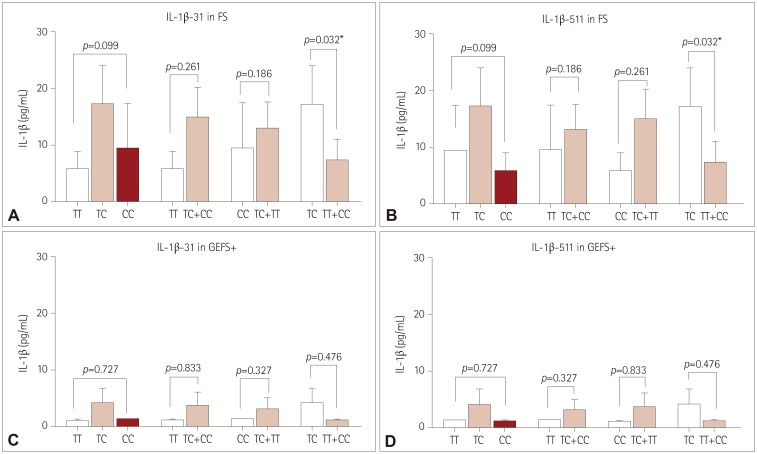
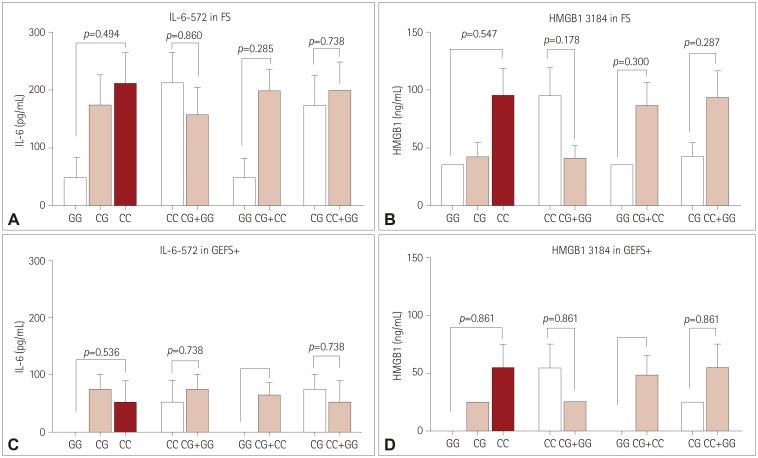
Near-complete linkage disequilibrium exists between IL-1β-31 and IL-1β-511 variants. CT genotypes of these variants were associated with significantly higher postictal serum IL-1β levels than were CC+TT genotypes in FS (both p<0.05). CT genotypes of IL-1β-31 and IL-1β-511 variants were more strongly associated with FS than were CC+TT genotypes (odds ratio=1.691 and 1.731, respectively). For GEFS+, serum IL-1β levels after AFS for CT genotypes of IL-1β-31 and IL-1β-511 were also higher than for CC+TT genotypes. No significant associations were found for IL-6 and HMGB1.
CONCLUSIONS
Genetic variants located in IL-1β-31 and IL-1β-511 promotor regions are correlated with higher postictal IL-1β levels in FS. These results suggest that IL-1 gene cluster variants in IL-1β-31 and IL-1β-511 are a host genetic factor for provoking FS in Korean children.
Keyword
MeSH Terms
-
Alleles
Child*
Enzyme-Linked Immunosorbent Assay
Epilepsy
Epilepsy, Generalized*
Fever
Genetic Predisposition to Disease
Genotype
HMGB1 Protein*
Humans
Inflammation
Interleukin-1
Interleukin-6*
Interleukins
Linkage Disequilibrium
Multigene Family
Promoter Regions, Genetic
Seizures
Seizures, Febrile*
HMGB1 Protein
Interleukin-1
Interleukin-6
Interleukins
Figure
Reference
-
1. Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994; 35 Suppl 2:S1–S6.
Article2. Millichap JG. Studies in febrile seizures. I. Height of body temperature as a measure of the febrile-seizure threshold. Pediatrics. 1959; 23:76–85. PMID: 13613867.3. Scheffer IE, Berkovic SF. Generalized epilepsy with febrile seizures plus. A genetic disorder with heterogeneous clinical phenotypes. Brain. 1997; 120:479–490. PMID: 9126059.
Article4. Myers KA, Scheffer IE, Berkovic SF, Commission IG. ILAE Genetics Commission. Genetic literacy series: genetic epilepsy with febrile seizures plus. Epileptic Disord. 2018; 20:232–238. PMID: 30078767.
Article5. Zhang YH, Burgess R, Malone JP, Glubb GC, Helbig KL, Vadlamudi L, et al. Genetic epilepsy with febrile seizures plus: refining the spectrum. Neurology. 2017; 89:1210–1219. PMID: 28842445.6. Choi J, Min HJ, Shin JS. Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. J Neuroinflammation. 2011; 8:135. PMID: 21989210.
Article7. Shi LM, Chen RJ, Zhang H, Jiang CM, Gong J. Cerebrospinal fluid neuron specific enolase, interleukin-1β and erythropoietin concentrations in children after seizures. Childs Nerv Syst. 2017; 33:805–811. PMID: 28236069.
Article8. Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. 2018; 15:144. PMID: 29764485.
Article9. Virta M, Hurme M, Helminen M. Increased frequency of interleukin 1β (-511) allele 2 in febrile seizures. Pediatr Neurol. 2002; 26:192–195. PMID: 11955925.10. Kanemoto K, Kawasaki J, Yuasa S, Kumaki T, Tomohiro O, Kaji R, et al. Increased frequency of Interleukin-1β-511T allele in patients with temporal lobe epilepsy, hippocampal sclerosis, and prolonged febrile convulsion. Epilepsia. 2003; 44:796–799. PMID: 12790892.
Article11. Yu X, Zhang N, Liu S, Xi Z, Zhang Y. Polymorphisms in the interleukin-1β (IL-1B) and interleukin-1α (IL-1A) genes on risk of febrile seizures: a meta-analysis. Neurol Sci. 2018; 39:1529–1536. PMID: 29808330.
Article12. Tilgen N, Pfeiffer H, Cobilanschi J, Rau B, Horvath S, Elger CE, et al. Association analysis between the human interleukin 1β (-511) gene polymorphism and susceptibility to febrile convulsions. Neurosci Lett. 2002; 334:68–70. PMID: 12431777.
Article13. Chou IC, Lin WD, Wang CH, Tsai CH, Li TC, Tsai FJ. Interleukin (IL)-1β, IL-1 receptor antagonist, IL-6, IL-8, IL-10, and tumor necrosis factor α gene polymorphisms in patients with febrile seizures. J Clin Lab Anal. 2010; 24:154–159. PMID: 20486195.
Article14. Capovilla G, Mastrangelo M, Romeo A, Vigevano F. Recommendations for the management of “febrile seizures”: Ad Hoc Task Force of LICE Guidelines Commission. Epilepsia. 2009; 50 Suppl 1:2–6.
Article15. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017; 58:512–521. PMID: 28276062.16. Yamada S, Inoue K, Yakabe K, Imaizumi H, Maruyama I. High mobility group protein 1 (HMGB1) quantified by ELISA with a monoclonal antibody that does not cross-react with HMGB2. Clin Chem. 2003; 49:1535–1537. PMID: 12928240.
Article17. Kwon JS, Joo YH, Nam HJ, Lim M, Cho EY, Jung MH, et al. Association of the glutamate transporter gene SLC1A1 with atypical antipsychotics-induced obsessive-compulsive symptoms. Arch Gen Psychiatry. 2009; 66:1233–1241. PMID: 19884611.
Article18. Xiong ZQ, Qian W, Suzuki K, McNamara JO. Formation of complement membrane attack complex in mammalian cerebral cortex evokes seizures and neurodegeneration. J Neurosci. 2003; 23:955–960. PMID: 12574424.
Article19. Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, et al. Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003; 23:8692–8700. PMID: 14507968.
Article20. Roseti C, Van Vliet EA, Cifelli P, Ruffolo G, Baayen JC, Di Castro MA, et al. GABAA currents are decreased by IL-1β in epileptogenic tissue of patients with temporal lobe epilepsy: implications for ictogenesis. Neurobiol Dis. 2015; 82:311–320. PMID: 26168875.
Article21. Dubé C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1β contributes to the generation of experimental febrile seizures. Ann Neurol. 2005; 57:152–155. PMID: 15622539.
Article22. Cho JH, Choi JS, Chun SW, Lee S, Han KJ, Kim HM. The IL-1B genetic polymorphism is associated with aspirin-induced Peptic Ulcers in a Korean Ethnic Group. Gut Liver. 2016; 10:362–368. PMID: 26601827.
Article23. Shahrokhi A, Zare-Shahabadi A, Soltani S, Ashrafi MR, Zoghi S, Hosseini SA, et al. Association of IL6 single nucleotide polymorphisms with febrile seizures. J Neurol Sci. 2014; 342:25–28. PMID: 24834995.
Article24. Nur BG, Sahinturk D, Coskun M, Duman O, Yavuzer U, Haspolat S. Single nucleotide polymorphism and production of IL-1β and IL-10 cytokines in febrile seizures. Neuropediatrics. 2012; 43:194–200. PMID: 22911481.
Article25. El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000; 404:398–402. PMID: 10746728.
Article26. Al Morshedy S, Elsaadany HF, Ibrahim HE, Sherif AM, Farghaly MA, Allah MA, et al. Interleukin-1β and interleukin-1receptor antagonist polymorphisms in Egyptian children with febrile seizures: a case-control study. Medicine (Baltimore). 2017; 96:e6370. PMID: 28296777.27. Diamond ML, Ritter AC, Failla MD, Boles JA, Conley YP, Kochanek PM, et al. IL-1β associations with posttraumatic epilepsy development: a genetics and biomarker cohort study. Epilepsia. 2014; 55:1109–1119. PMID: 24754437.
Article28. Hall SK, Perregaux DG, Gabel CA, Woodworth T, Durham LK, Huizinga TW, et al. Correlation of polymorphic variation in the promoter region of the interleukin-1β gene with secretion of interleukin-1β protein. Arthritis Rheum. 2004; 50:1976–1983. PMID: 15188375.
Article29. Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, et al. Tolllike receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010; 16:413–419. PMID: 20348922.
Article30. Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011; 7:31–40. PMID: 21135885.
Article31. Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008; 180:2531–2537. PMID: 18250463.
Article32. Youn JH, Oh YJ, Kim ES, Choi JE, Shin JS. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-α production in human monocytes. J Immunol. 2008; 180:5067–5074. PMID: 18354232.
Article33. Chayka O, Kintscher J, Braas D, Klempnauer KH. v-Myb mediates cooperation of a cell-specific enhancer with the mim-1 promoter. Mol Cell Biol. 2005; 25:499–511. PMID: 15601869.34. Bi D, Chen M, Zhang X, Wang H, Xia L, Shang Q, et al. The association between sex-related interleukin-6 gene polymorphisms and the risk for cerebral palsy. J Neuroinflammation. 2014; 11:100. PMID: 24903966.
Article35. Fornage M, Chiang YA, O'Meara ES, Psaty BM, Reiner AP, Siscovick DS, et al. Biomarkers of inflammation and MRI-defined small vessel disease of the brain: the Cardiovascular Health Study. Stroke. 2008; 39:1952–1959. PMID: 18436879.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Selection of High Risk Group According to Risk Factors of Recurrent Febrile Seizures
- Comparison of Serum Zinc Levels Measured by Inductively Coupled Plasma Mass Spectrometry in Preschool Children with Febrile and Afebrile Seizures
- Clinical Review of the Development Epilepsy in Patients with Febrile Seizure
- A Study on the Clinical Features and the Predictors of Febrile Seizure Plus
- Epilepsy in children with a history of febrile seizures