J Clin Neurol.
2019 Oct;15(4):511-516. 10.3988/jcn.2019.15.4.511.
Prediction of Chemotherapy-Induced Peripheral Neuropathy in Patients with Lymphoma and Myeloma: the Roles of Brain-Derived Neurotropic Factor Protein Levels and A Gene Polymorphism
- Affiliations
-
- 1Hematology Unit and Laboratories, Galilee Medical Center, Naharia, Israel. davidA@GMC.gov.il
- 2Azrieli Faculty of Medicine, Bar-Ilan University, Safed, Israel.
- 3Department of Hematology and Bone Marrow Transplantation, Rambam Health Care Campus, Haifa, Israel.
- 4The Ruth and Bruce Rappaport Faculty of Medicine, Technion, Israel Institute of Technology, Haifa, Israel.
- KMID: 2467761
- DOI: http://doi.org/10.3988/jcn.2019.15.4.511
Abstract
- BACKGROUND AND PURPOSE
Brain-derived neurotrophic factor (BDNF) is a neuronal growth factor that plays an essential role in the maintenance of the nervous system. We have evaluated the peripheral blood protein levels of BDNF and the valine-to-methionine substitution at codon 66 (Val66Met) single-nucleotide polymorphism (SNP) as potential biomarkers for the early recognition of chemotherapy-induced peripheral neuropathy (CIPN) in non-Hodgkin lymphoma and multiple myeloma patients.
METHODS
CIPN was assessed in 45 patients at the diagnosis and during vincristine or bortezomib-based therapy using objective [reduced version of the Total Neuropathy Score (TNSr)] and subjective (FACT-GOG-NTx) tools. Depression was assessed using the Patient Health Questionnaire-9 (PHQ-9) questionnaire. BDNF protein levels and the Val66Met SNP were determined using ELISA and Sanger sequencing.
RESULTS
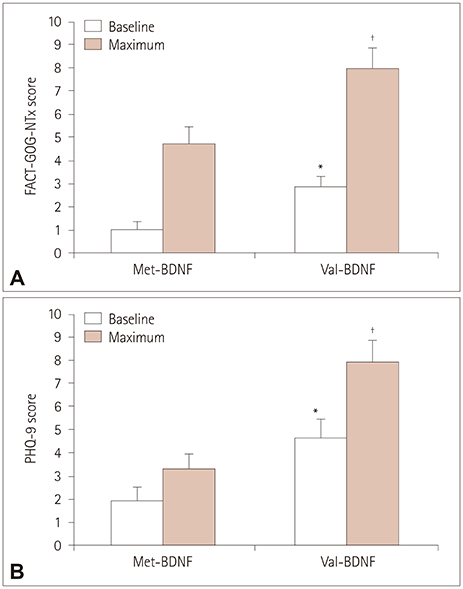
The pretreatment BDNF protein level was inversely correlated with the maximum TNSr, FACT-GOG-NTx, and PHQ-9 scores in both genotypes. BDNF patients with the Val/Val genotype demonstrated significantly higher maximum FACT-GOG-NTx and PHQ-9 scores than those with the Val/Met and Met/Met genotypes (Met-BNDF carriers). Correlations between PHQ-9 and TNSr score were found only in Met-BDNF carriers, suggesting that peripheral neuropathy and depression coincide in Met-BDNF carriers.
CONCLUSIONS
Determining the BDNF protein levels before initiating chemotherapy might be a useful tool for CIPN risk assessment and preemptive dose modification. The present data should be validated in larger studies that include other neurotoxic agents.
Keyword
MeSH Terms
-
Biomarkers
Brain-Derived Neurotrophic Factor
Codon
Depression
Diagnosis
Drug Therapy
Enzyme-Linked Immunosorbent Assay
Genes, vif*
Genotype
Humans
Lymphoma*
Lymphoma, Non-Hodgkin
Multiple Myeloma
Nervous System
Neurons
Peripheral Nervous System Diseases*
Risk Assessment
Vincristine
Biomarkers
Brain-Derived Neurotrophic Factor
Codon
Vincristine
Figure
Reference
-
1. Rocamora N, García-Ladona FJ, Palacios JM, Mengod G. Differential expression of brain-derived neurotrophic factor, neurotrophin-3, and low-affinity nerve growth factor receptor during the postnatal development of the rat cerebellar system. Brain Res Mol Brain Res. 1993; 17:1–8.
Article2. Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci. 2007; 1122:130–143.
Article3. Novikova L, Novikov L, Kellerth JO. Brain-derived neurotrophic factor reduces necrotic zone and supports neuronal survival after spinal cord hemisection in adult rats. Neurosci Lett. 1996; 220:203–206.
Article4. Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activitydependent secretion of BDNF and human memory and hippocampal function. Cell. 2003; 112:257–269.
Article5. Białecka M, Kurzawski M, Roszmann A, Robowski P, Sitek EJ, Honczarenko K, et al. BDNF G196A (Val66Met) polymorphism associated with cognitive impairment in Parkinson's disease. Neurosci Lett. 2014; 561:86–90.
Article6. Borroni B, Archetti S, Costanzi C, Grassi M, Ferrari M, Radeghieri A, et al. Role of BDNF Val66Met functional polymorphism in Alzheimer’s disease-related depression. Neurobiol Aging. 2009; 30:1406–1412.
Article7. Azoulay D, Urshansky N, Karni A. Low and dysregulated BDNF secretion from immune cells of MS patients is related to reduced neuroprotection. J Neuroimmunol. 2008; 195:186–193.
Article8. Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999; 189:865–870.
Article9. Azoulay D, Vachapova V, Shihman B, Miler A, Karni A. Lower brainderived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. J Neuroimmunol. 2005; 167:215–218.
Article10. Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002; 87:728–734.
Article11. Yamamoto H, Gurney ME. Human platelets contain brain-derived neurotrophic factor. J Neurosci. 1990; 10:3469–3478.
Article12. Chacón-Fernández P, Säuberli K, Colzani M, Moreau T, Ghevaert C, Barde YA. Brain-derived neurotrophic factor in megakaryocytes. J Biol Chem. 2016; 291:9872–9881.
Article13. Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014; 155:2461–2470.
Article14. Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 2003; 63:1549–1563.
Article15. Cavaletti G, Bogliun G, Marzorati L, Zincone A, Piatti M, Colombo N, et al. Early predictors of peripheral neurotoxicity in cisplatin and paclitaxel combination chemotherapy. Ann Oncol. 2004; 15:1439–1442.
Article16. Andreassen CS, Jakobsen J, Flyvbjerg A, Andersen H. Expression of neurotrophic factors in diabetic muscle--relation to neuropathy and muscle strength. Brain. 2009; 132:2724–2733.
Article17. Azoulay D, Leibovici A, Sharoni R, Shaoul E, Gross B, Braester A, et al. Association between Met-BDNF allele and vulnerability to paclitaxel-induced peripheral neuropathy. Breast Cancer Res Treat. 2015; 153:703–704.
Article18. Azoulay D, Lavie D, Horowitz N, Suriu C, Gatt ME, Akria L, et al. Bortezomib-induced peripheral neuropathy is related to altered levels of brain-derived neurotrophic factor in the peripheral blood of patients with multiple myeloma. Br J Haematol. 2014; 164:454–456.
Article19. Azoulay D, Nasser R, Sharon R, Simanovich L, Akria L, Shaoul E, et al. Brain derived neurotropic factor single nucleotide polymorphism Val-66Met and serum protein levels are associated with development of vincristine-induced peripheral neuropathy in patients with lymphoma. Br J Haematol. 2019; 185:175–177.
Article20. Smith EM, Beck SL, Cohen J. The total neuropathy score: a tool for measuring chemotherapy-induced peripheral neuropathy. Oncol Nurs Forum. 2008; 35:96–102.
Article21. Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007; 22:1596–1602.
Article22. Pliego-Rivero FB, Bayatti N, Giannakoulopoulos X, Glover V, Bradford HF, Stern G, et al. Brain-derived neurotrophic factor in human platelets. Biochem Pharmacol. 1997; 54:207–209.
Article23. Tamura S, Suzuki H, Hirowatari Y, Hatase M, Nagasawa A, Matsuno K, et al. Release reaction of brain-derived neurotrophic factor (BDNF) through PAR1 activation and its two distinct pools in human platelets. Thromb Res. 2011; 128:e55–e61.
Article24. Chen ZY, Bath K, McEwen B, Hempstead B, Lee F. Impact of genetic variant BDNF (Val66Met) on brain structure and function. Novartis Found Symp. 2008; 289:180–188.
Article25. Ng T, Teo SM, Yeo HL, Shwe M, Gan YX, Cheung YT, et al. Brain-derived neurotrophic factor genetic polymorphism (rs6265) is protective against chemotherapy-associated cognitive impairment in patients with early-stage breast cancer. Neuro Oncol. 2016; 18:244–251.
Article26. Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008; 11:1169–1180.
Article27. Yu H, Chen ZY. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol Sin. 2011; 32:3–11.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Potential of histone deacetylase 6 inhibitors in alleviating chemotherapy-induced peripheral neuropathy
- Association between the Brain-Derived Neurotropic Factor and Attention Deficit Hyperactivity Disorder
- Peripheral Neuropathy and Pain Caused by Cancer Chemotherapy
- A Case of Coincident Multiple Myeloma and Nasal NK/T-Cell Lymphoma
- Myricetin prevents sleep deprivation-induced cognitive impairment and neuroinflammation in rat brain via regulation of brain-derived neurotropic factor