Tuberc Respir Dis.
2020 Jan;83(1):61-70. 10.4046/trd.2019.0048.
Circulating Tumor Cell Number Is Associated with Primary Tumor Volume in Patients with Lung Adenocarcinoma
- Affiliations
-
- 1Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea. drahnjj@gmail.com
- 2Liquid Biopsy and Precision Medicine Division, Clinomics Inc., Ulsan, Korea. drahnjj@gmail.com
- 3Department of Radiology, Ulsan University Hospital, Ulsan, Korea.
- KMID: 2466621
- DOI: http://doi.org/10.4046/trd.2019.0048
Abstract
- BACKGROUND
Circulating tumor cells (CTCs) are frequently detected in patients with advanced-stage malignant tumors and could act as a predictor of poor prognosis. However, there is a paucity of data on the relationship between CTC number and primary tumor volume in patients with lung cancer. Therefore, our study aimed to evaluate the relationship between CTC number and primary tumor volume in patients with lung adenocarcinoma.
METHODS
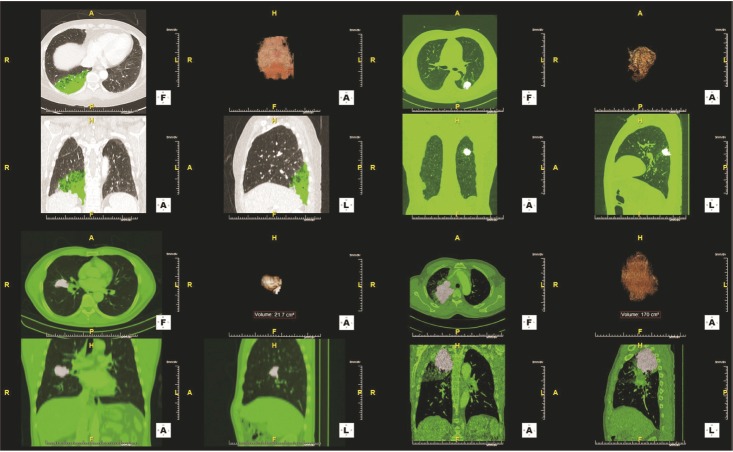
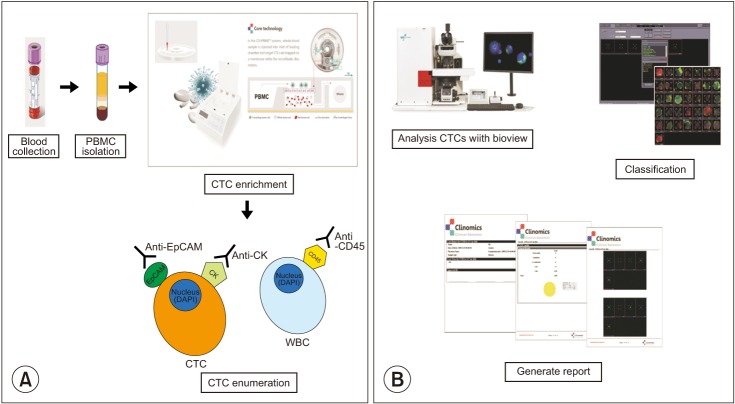
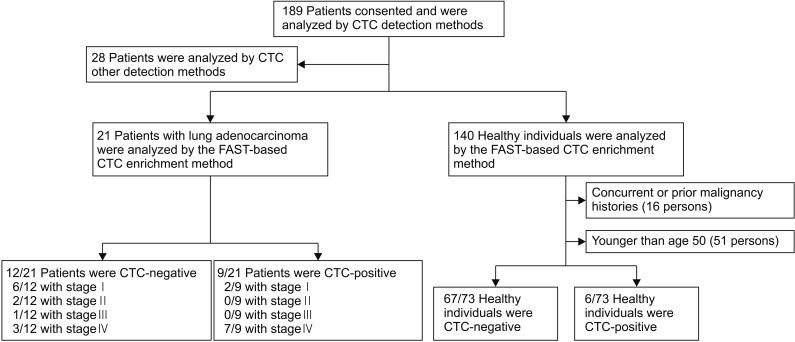
We collected blood samples from 21 patients with treatment-naive lung adenocarcinoma and 73 healthy individuals. To count CTCs, we used a CTC enrichment method based on fluid-assisted separation technology. We compared CTC numbers between lung adenocarcinoma patients and healthy individuals using propensity score matching, and performed linear regression analysis to analyze the relationship between CTC number and primary tumor volume in lung adenocarcinoma patients.
RESULTS
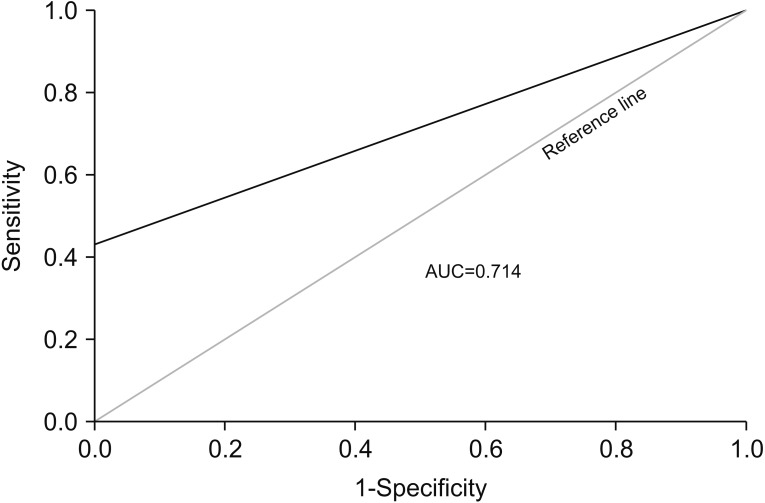
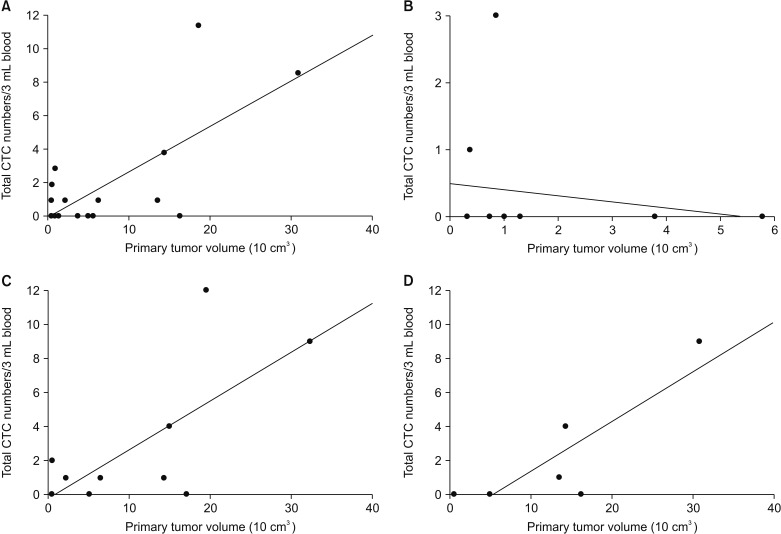
CTC positivity was significantly more common in lung adenocarcinoma patients than in healthy individuals (p<0.001). The median primary tumor volume in CTC-negative and CTC-positive patients was 10.0 cm³ and 64.8 cm³, respectively. Multiple linear regression analysis showed that the number of CTCs correlated with primary tumor volume in lung adenocarcinoma patients (β=0.903, p=0.002). Further subgroup analysis showed a correlation between CTC number and primary tumor volume in patients with distant (p=0.024) and extra-thoracic (p=0.033) metastasis (not in patients with distant metastasis).
CONCLUSION
Our study showed that CTC numbers may be associated with primary tumor volume in lung adenocarcinomas patients, especially in those with distant metastasis.
MeSH Terms
Figure
Reference
-
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108. PMID: 25651787.
Article2. Zhou J, Dong F, Cui F, Xu R, Tang X. The role of circulating tumor cells in evaluation of prognosis and treatment response in advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2017; 79:825–833. PMID: 28289866.
Article3. Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014; 84:13–22. PMID: 24524818.
Article4. O'Flaherty L, Wikman H, Pantel K. Biology and clinical significance of circulating tumor cell subpopulations in lung cancer. Transl Lung Cancer Res. 2017; 6:431–443. PMID: 28904887.5. Yap TA, Lorente D, Omlin A, Olmos D, de Bono JS. Circulating tumor cells: a multifunctional biomarker. Clin Cancer Res. 2014; 20:2553–2568. PMID: 24831278.
Article6. Han Y, Su C, Liu Z. Methods for detection of circulating cells in non-small cell lung cancer. Front Biosci (Landmark Ed). 2014; 19:896–903. PMID: 24896324.
Article7. Karachaliou N, Mayo-de-Las-Casas C, Molina-Vila MA, Rosell R. Real-time liquid biopsies become a reality in cancer treatment. Ann Transl Med. 2015; 3:36. PMID: 25815297.8. Chen Q, Ge F, Cui W, Wang F, Yang Z, Guo Y, et al. Lung cancer circulating tumor cells isolated by the EpCAM-independent enrichment strategy correlate with cytokeratin 19-derived CYFRA21-1 and pathological staging. Clin Chim Acta. 2013; 419:57–61. PMID: 23415723.
Article9. Tanaka F, Yoneda K, Kondo N, Hashimoto M, Takuwa T, Matsumoto S, et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res. 2009; 15:6980–6986. PMID: 19887487.
Article10. Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011; 29:1556–1563. PMID: 21422424.
Article11. Lindsay CR, Faugeroux V, Michiels S, Pailler E, Facchinetti F, Ou D, et al. A prospective examination of circulating tumor cell profiles in non-small-cell lung cancer molecular subgroups. Ann Oncol. 2017; 28:1523–1531. PMID: 28633480.
Article12. Kim TH, Lim M, Park J, Oh JM, Kim H, Jeong H, et al. FAST: size-selective, clog-free isolation of rare cancer cells from whole blood at a liquid-liquid interface. Anal Chem. 2017; 89:1155–1162. PMID: 27958721.
Article13. Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999; 150:327–333. PMID: 10453808.
Article14. Kim SH, Hong SB, Yun SC, Choi WI, Ahn JJ, Lee YJ, et al. Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: analytic strategy using propensity scores. Am J Respir Crit Care Med. 2011; 183:1207–1214. PMID: 21471084.15. Sonn CH, Cho JH, Kim JW, Kang MS, Lee J, Kim J. Detection of circulating tumor cells in patients with non-small cell lung cancer using a size-based platform. Oncol Lett. 2017; 13:2717–2722. PMID: 28454457.
Article16. Katoh M, Neumaier M, Nezam R, Izbicki JR, Schumacher U. Correlation of circulating tumor cells with tumor size and metastatic load in a spontaneous lung metastasis model. Anticancer Res. 2004; 24:1421–1425. PMID: 15274304.17. Delfau-Larue MH, van der Gucht A, Dupuis J, Jais JP, Nel I, Beldi-Ferchiou A, et al. Total metabolic tumor volume, circulating tumor cells, cell-free DNA: distinct prognostic value in follicular lymphoma. Blood Adv. 2018; 2:807–816. PMID: 29636326.
Article18. Fu L, Zhu Y, Jing W, Guo D, Kong L, Yu J. Incorporation of circulating tumor cells and whole-body metabolic tumor volume of (18)F-FDG PET/CT improves prediction of outcome in IIIB stage small-cell lung cancer. Chin J Cancer Res. 2018; 30:596–604. PMID: 30700928.
Article19. Lopez-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of metastasis by NK cells. Cancer Cell. 2017; 32:135–154. PMID: 28810142.
Article20. Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017; 17:302–317. PMID: 28303905.
Article21. Hosokawa M, Kenmotsu H, Koh Y, Yoshino T, Yoshikawa T, Naito T, et al. Size-based isolation of circulating tumor cells in lung cancer patients using a microcavity array system. PLoS One. 2013; 8:e67466. PMID: 23840710.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tripe synchronous primary lung cancer: one case report
- Clinical Application of Circulating Tumor DNA Analysis
- Circulating Tumor Cell and Cell-free Circulating Tumor DNA in Lung Cancer
- Diagnostic Usefulness of Simultaneous Measurement ofSerum Tumor Markers in Lung Cancer Patients
- Primary Signet Ring Cell Carcinoma of the Lung: Report of Two Cases