Blood Res.
2019 Dec;54(4):274-281. 10.5045/br.2019.54.4.274.
Clinical significance of T cell receptor excision circle (TREC) quantitation after allogenic HSCT
- Affiliations
-
- 1Clinical Pathology Department, Alexandria Faculty of Medicine, Alexandria, Egypt. Neveen.Lewis@alexmed.edu.eg
- 2Clinical Hematology Department, Head of BMT Unit, Alexandria Faculty of Medicine, Alexandria, Egypt.
- KMID: 2466593
- DOI: http://doi.org/10.5045/br.2019.54.4.274
Abstract
- BACKGROUND
Hematopoietic stem cell transplantation (HSCT) is a well-established treatment modality for a variety of diseases. Immune reconstitution is an important event that determines outcomes. The immune recovery of T cells relies on peripheral expansion of mature graft cells, followed by differentiation of donor-derived hematopoietic stem cells. The formation of new T cells occurs in the thymus and as a byproduct, T cell receptor excision circles (TRECs) are released. Detection of TRECs by PCR is a reliable method for estimating the amount of newly formed T cells in the circulation and, indirectly, for estimating thymic function. The aim of this study was to determine the role of TREC quantitation in predicting outcomes of human leucocyte antigen (HLA) identical allogenic HSCT.
METHODS
The study was conducted on 100 patients receiving allogenic HSCT from an HLA identical sibling. TREC quantification was done by real time PCR using a standard curve.
RESULTS
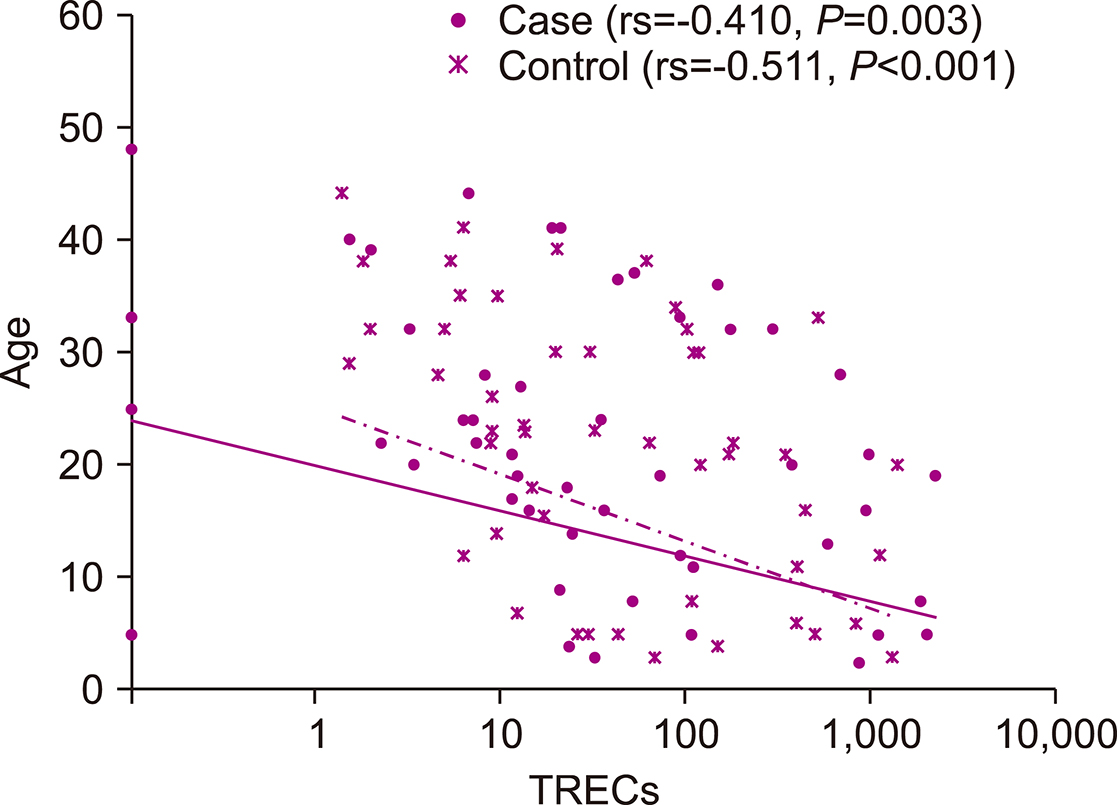
TREC levels were inversely related to age (P=0.005) and were significantly lower in patients with malignant diseases than in those with benign diseases (P=0.038). TREC levels could predict relapse as an outcome but not graft versus host disease (GvHD) and infections.
CONCLUSION
Age and nature of disease determine the TREC levels, which are related to relapse.
MeSH Terms
Figure
Reference
-
1. Toubert A, Glauzy S, Douay C, Clave E. Thymus and immune reconstitution after allogeneic hematopoietic stem cell transplantation in humans: never say never again. Tissue Antigens. 2012; 79:83–89.
Article2. van den Brink MR, Velardi E, Perales MA. Immune reconstitution following stem cell transplantation. Hematology Am Soc Hematol Educ Program. 2015; 2015:215–219.
Article3. Ogonek J, Kralj Juric M, Ghimire S, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016; 7:507.
Article4. Chaudhry MS, Velardi E, Malard F, van den Brink MR. Immune reconstitution after allogeneic hematopoietic stem cell transplantation: time to t up the thymus. J Immunol. 2017; 198:40–46.
Article5. de Koning C, Plantinga M, Besseling P, Boelens JJ, Nierkens S. Immune reconstitution after allogeneic hematopoietic cell transplantation in children. Biol Blood Marrow Transplant. 2016; 22:195–206.
Article6. van der Spek J, Groenwold RH, van der Burg M, van Montfrans JM. TREC based newborn screening for severe combined immunodeficiency disease: a systematic review. J Clin Immunol. 2015; 35:416–430.
Article7. Yamanaka K, Yawalkar N, Jones DA, et al. Decreased T-cell receptor excision circles in cutaneous T-cell lymphoma. Clin Cancer Res. 2005; 11:5748–5755.
Article8. Serana F, Chiarini M, Zanotti C, et al. Use of V(D)J recombination excision circles to identify T- and B-cell defects and to monitor the treatment in primary and acquired immunodeficiencies. J Transl Med. 2013; 11:119.
Article9. Drylewicz J, Vrisekoop N, Mugwagwa T, et al. Reconciling longitudinal naive T-cell and TREC dynamics during HIV-1 infection. PLoS One. 2016; 11:e0152513.
Article10. Bamoulid J, Courivaud C, Crepin T, et al. Pretransplant thymic function predicts acute rejection in antithymocyte globulin-treated renal transplant recipients. Kidney Int. 2016; 89:1136–1143.
Article11. Morgun A, Shulzhenko N, Socorro-Silva A, Diniz RV, Almeida DR, Gerbase-Delima M. T cell receptor excision circles (TRECs) in relation to acute cardiac allograft rejection. J Clin Immunol. 2004; 24:612–616.
Article12. Weinberg K, Blazar BR, Wagner JE, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001; 97:1458–1466.
Article13. Przybylski GK, Kreuzer KA, Siegert W, Schmidt CA. No recovery of T-cell receptor excision circles (TRECs) after non-myeloablative allogeneic hematopoietic stem cell transplantation is correlated with the onset of GvHD. J Appl Genet. 2007; 48:397–404.
Article14. Gaballa A, Sundin M, Stikvoort A, et al. T cell receptor excision circle (TREC) monitoring after allogeneic stem cell transplantation; a predictive marker for complications and clinical outcome. Int J Mol Sci. 2016; 17:E1705.
Article15. Gaballa A, Norberg A, Stikvoort A, et al. Assessment of TREC, KREC and telomere length in long-term survivors after allogeneic HSCT: the role of GvHD and graft source and evidence for telomere homeostasis in young recipients. Bone Marrow Transplant. 2018; 53:69–77.
Article16. Lorenzi AR, Patterson AM, Pratt A, et al. Determination of thymic function directly from peripheral blood: a validated modification to an established method. J Immunol Methods. 2008; 339:185–194.
Article17. Eyrich M, Wollny G, Tzaribaschev N, et al. Onset of thymic recovery and plateau of thymic output are differentially regulated after stem cell transplantation in children. Biol Blood Marrow Transplant. 2005; 11:194–205.
Article18. Jiménez M, Martínez C, Ercilla G, et al. Reduced-intensity conditioning regimen preserves thymic function in the early period after hematopoietic stem cell transplantation. Exp Hematol. 2005; 33:1240–1248.
Article19. Fu YW, Wu DP, Cen JN, et al. Patterns of T-cell reconstitution by assessment of T-cell receptor excision circle and T-cell receptor clonal repertoire after allogeneic hematopoietic stem cell transplantation in leukemia patients--a study in Chinese patients. Eur J Haematol. 2007; 79:138–145.
Article20. Ringhoffer S, Rojewski M, Döhner H, Bunjes D, Ringhoffer M. T-cell reconstitution after allogeneic stem cell transplantation: assessment by measurement of the sjTREC/βTREC ratio and thymic naive T cells. Haematologica. 2013; 98:1600–1608.
Article21. Weinberg K, Blazar BR, Wagner JE, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001; 97:1458–1466.
Article22. Velardi E, Dudakov JA, van den Brink MR. Clinical strategies to enhance thymic recovery after allogeneic hematopoietic stem cell transplantation. Immunol Lett. 2013; 155:31–35.
Article23. Krenger W, Blazar BR, Holländer GA. Thymic T-cell development in allogeneic stem cell transplantation. Blood. 2011; 117:6768–6776.
Article24. Svaldi M, Lanthaler AJ, Dugas M, et al. T-cell receptor excision circles: a novel prognostic parameter for the outcome of transplantation in multiple myeloma patients. Br J Haematol. 2003; 122:795–801.
Article25. Li Y, Yin Q, Yang L, et al. Reduced levels of recent thymic emigrants in acute myeloid leukemia patients. Cancer Immunol Immunother. 2009; 58:1047–1055.
Article26. Haining WN, Neuberg DS, Keczkemethy HL, et al. Antigen-specific T-cell memory is preserved in children treated for acute lymphoblastic leukemia. Blood. 2005; 106:1749–1754.
Article27. Williams KM, Mella H, Lucas PJ, Williams JA, Telford W, Gress RE. Single cell analysis of complex thymus stromal cell populations: rapid thymic epithelia preparation characterizes radiation injury. Clin Transl Sci. 2009; 2:279–285.
Article28. Clave E, Rocha V, Talvensaari K, et al. Prognostic value of pretransplantation host thymic function in HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2005; 105:2608–2613.
Article29. Saglio F, Cena S, Berger M, et al. Association between thymic function and allogeneic hematopoietic stem cell transplantation outcome: results of a pediatric study. Biol Blood Marrow Transplant. 2015; 21:1099–1105.
Article30. Törlén J, Gaballa A, Remberger M, et al. Effect of graft-versus-host disease prophylaxis regimens on T and B cell reconstitution after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019; 25:1260–1268.
Article31. Krenger W, Schmidlin H, Cavadini G, Holländer GA. On the relevance of TCR rearrangement circles as molecular markers for thymic output during experimental graft-versus-host disease. J Immunol. 2004; 172:7359–7367.
Article32. Wu X, Zhu K, Du X, et al. Frequency analysis of TRBV subfamily sjTRECs to characterize T-cell reconstitution in acute leukemia patients after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. 2011; 4:19.
Article33. Bamoulid J, Courivaud C, Crepin T, et al. Pretransplant thymic function predicts acute rejection in antithymocyte globulin-treated renal transplant recipients. Kidney Int. 2016; 89:1136–1143.
Article34. Skert C, Perucca S, Chiarini M, et al. Sequential monitoring of lymphocyte subsets and of T-and-B cell neogenesis indexes to identify time-varying immunologic profiles in relation to graft-versus-host disease and relapse after allogeneic stem cell transplantation. PLoS One. 2017; 12:e0175337.
Article35. Wils EJ, van der Holt B, Broers AE, et al. Insufficient recovery of thymopoiesis predicts for opportunistic infections in allogeneic hematopoietic stem cell transplant recipients. Haematologica. 2011; 96:1846–1854.
Article36. da Rocha LKA, Freschi de Barros S, Bandeira F, et al. Thymopoiesis in pre- and post-hematopoietic stem cell transplantation. Front Immunol. 2018; 9:1889.37. Uzunel M, Sairafi D, Remberger M, Mattsson J, Uhlin M. T-cell receptor excision circle levels after allogeneic stem cell transplantation are predictive of relapse in patients with acute myeloid leukemia and myelodysplastic syndrome. Stem Cells Dev. 2014; 23:1559–1567.
Article38. Sairafi D, Mattsson J, Uhlin M, Uzunel M. Thymic function after allogeneic stem cell transplantation is dependent on graft source and predictive of long term survival. Clin Immunol. 2012; 142:343–350.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The First Newborn Screening Study of T-Cell Receptor Excision Circle and κ- Deleting Recombination Excision Circle for Severe Combined Immunodeficiency in Korea: A Pilot Study
- Bronchiolitis Obliterans Syndrome after Allogenic Hematopoietic Stem Cell Transplantation in Pediatric Patients
- Significance of Epstein-Barr Virus DNA Quantitation in Donors of Hematopoietic Stem Cell Transplantation
- Case of Donor Cell Leukemia after Allogenic Bone Marrow Transplantation for Severe Aplastic Anemia
- Association of FOXP3 Single Nucleotide Polymorphisms With Clinical Outcomes After Allogenic Hematopoietic Stem Cell Transplantation