Korean J Physiol Pharmacol.
2020 Jan;24(1):111-119. 10.4196/kjpp.2020.24.1.111.
Decreased inward rectifier and voltage-gated K⺠currents of the right septal coronary artery smooth muscle cells in pulmonary arterial hypertensive rats
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 03080, Korea. physiolksj@gmail.com
- 2Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 03080, Korea.
- 3Department of Nursing, Chung-Ang University, Seoul 06974, Korea. hyoo@cau.ac.kr
- 4Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul 03080, Korea.
- KMID: 2466575
- DOI: http://doi.org/10.4196/kjpp.2020.24.1.111
Abstract
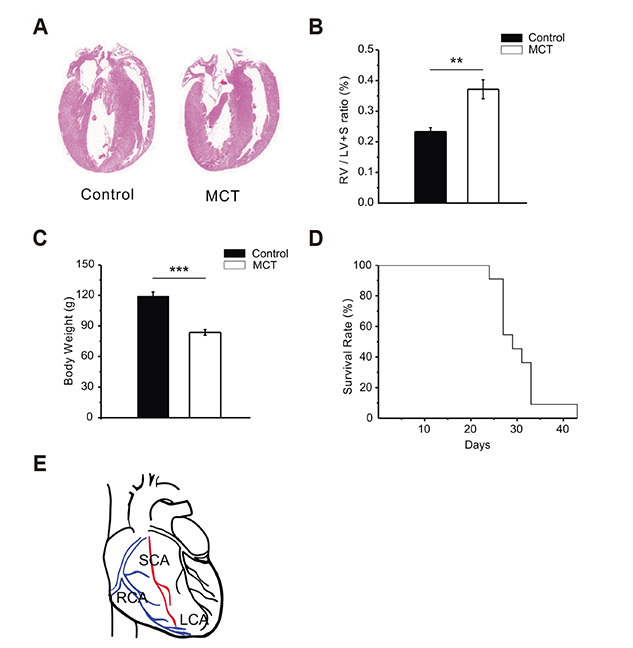
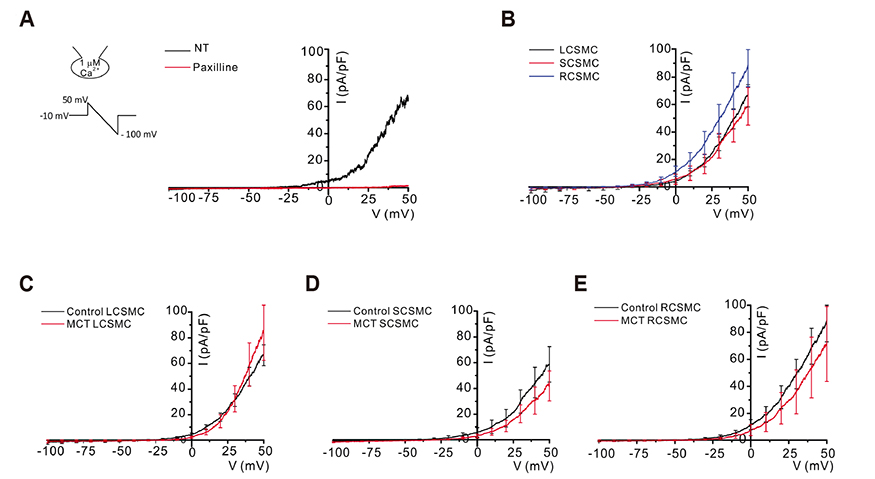
- In vascular smooth muscle, K⺠channels, such as voltage-gated K⺠channels (Kv), inward-rectifier K⺠channels (Kir), and big-conductance Ca²âº-activated K⺠channels (BK(Ca)), establish a hyperpolarized membrane potential and counterbalance the depolarizing vasoactive stimuli. Additionally, Kir mediates endothelium-dependent hyperpolarization and the active hyperemia response in various vessels, including the coronary artery. Pulmonary arterial hypertension (PAH) induces right ventricular hypertrophy (RVH), thereby elevating the risk of ischemia and right heart failure. Here, using the whole-cell patch-clamp technique, we compared Kv and Kir current densities (I(Kv) and I(Kir)) in the left (LCSMCs), right (RCSMCs), and septal branches of coronary smooth muscle cells (SCSMCs) from control and monocrotaline (MCT)-induced PAH rats exhibiting RVH. In control rats, (1) I(Kv) was larger in RCSMCs than that in SCSMCs and LCSMCs, (2) I(Kv) inactivation occurred at more negative voltages in SCSMCs than those in RCSMCs and LCSMCs, (3) I(Kir) was smaller in SCSMCs than that in RCSMCs and LCSMCs, and (4) I(BKCa) did not differ between branches. Moreover, in PAH rats, I(Kir) and I(Kv) decreased in SCSMCs, but not in RCSMCs or LCSMCs, and I(BKCa) did not change in any of the branches. These results demonstrated that SCSMC-specific decreases in I(Kv) and I(Kir) occur in an MCT-induced PAH model, thereby offering insights into the potential pathophysiological implications of coronary blood flow regulation in right heart disease. Furthermore, the relatively smaller I(Kir) in SCSMCs suggested a less effective vasodilatory response in the septal region to the moderate increase in extracellular K⺠concentration under increased activity of the myocardium.
Keyword
MeSH Terms
-
Animals
Coronary Vessels*
Heart Diseases
Heart Failure
Hyperemia
Hypertension
Hypertrophy, Right Ventricular
Ischemia
Membrane Potentials
Monocrotaline
Muscle, Smooth*
Muscle, Smooth, Vascular
Myocardium
Myocytes, Smooth Muscle*
Patch-Clamp Techniques
Potassium Channels
Rats*
Septum of Brain
Monocrotaline
Potassium Channels
Figure
Reference
-
1. Werner ME, Ledoux J. K+ channels in biological processes: vascular K+ channels in the regulation of blood pressure. J Receptor Ligand Channel Res. 2014; 7:51–60.2. Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995; 268(4 Pt 1):C799–C822.
Article3. An JR, Li H, Seo MS, Park WS. Inhibition of voltage-dependent K+ current in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic drug propafenone. Korean J Physiol Pharmacol. 2018; 22:597–605.4. Knot HJ, Zimmermann PA, Nelson MT. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J Physiol. 1996; 492(Pt 2):419–430.5. Longden TA, Nelson MT. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation. 2015; 22:183–196.6. Dopico AM, Bukiya AN, Jaggar JH. Calcium- and voltage-gated BK channels in vascular smooth muscle. Pflugers Arch. 2018; 470:1271–1289.
Article7. Gregg DE. The coronary circulation. Physiol Rev. 1946; 26:28–46.
Article8. Goodwill AG, Dick GM, Kiel AM, Tune JD. Regulation of coronary blood flow. Compr Physiol. 2017; 7:321–382.
Article9. Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002; 106:653–658.
Article10. van Wolferen SA, Marcus JT, Westerhof N, Spreeuwenberg MD, Marques KM, Bronzwaer JG, Henkens IR, Gan CT, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J. 2008; 29:120–127.
Article11. Kim HJ, Yoo HY. Hypoxic pulmonary vasoconstriction and vascular contractility in monocrotaline-induced pulmonary arterial hypertensive rats. Korean J Physiol Pharmacol. 2016; 20:641–647.
Article12. Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004; 351:1655–1665.
Article13. Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009; 297:L1013–L1032.
Article14. Rabinovitch M. Monocrotaline-induced pulmonary hypertension in rats. In : Simon DI, Rogers C, editors. Vascular disease and injury: preclinical research. Totowa: Humana Press;2001. p. 261–280.15. Boucherat O, Chabot S, Antigny F, Perros F, Provencher S, Bonnet S. Potassium channels in pulmonary arterial hypertension. Eur Respir J. 2015; 46:1167–1177.
Article16. Nakazawa H, Hori M, Ozaki H, Karaki H. Mechanisms underlying the impairment of endothelium-dependent relaxation in the pulmonary artery of monocrotaline-induced pulmonary hypertensive rats. Br J Pharmacol. 1999; 128:1098–1104.
Article17. Xie L, Lin P, Xie H, Xu C. Effects of atorvastatin and losartan on monocrotaline-induced pulmonary artery remodeling in rats. Clin Exp Hypertens. 2010; 32:547–554.
Article18. Meloche J, Lampron MC, Nadeau V, Maltais M, Potus F, Lambert C, Tremblay E, Vitry G, Breuils-Bonnet S, Boucherat O, Charbonneau E, Provencher S, Paulin R, Bonnet S. Implication of inflammation and epigenetic readers in coronary artery remodeling in patients with pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2017; 37:1513–1523.
Article19. Gautier M, Hyvelin JM, de Crescenzo V, Eder V, Bonnet P. Heterogeneous Kv1 function and expression in coronary myocytes from right and left ventricles in rats. Am J Physiol Heart Circ Physiol. 2007; 292:H475–H482.
Article20. Morales-Cano D, Moreno L, Barreira B, Pandolfi R, Chamorro V, Jimenez R, Villamor E, Duarte J, Perez-Vizcaino F, Cogolludo A. Kv7 channels critically determine coronary artery reactivity: leftright differences and down-regulation by hyperglycaemia. Cardiovasc Res. 2015; 106:98–108.
Article21. Hyvelin JM, Gautier M, Lemaire MC, Bonnet P, Eder V. Adaptative modifications of right coronary myocytes voltage-gated K+ currents in rat with hypoxic pulmonary hypertension. Pflugers Arch. 2009; 457:721–730.22. Lee S, Yang Y, Tanner MA, Li M, Hill MA. Heterogeneity in Kv7 channel function in the cerebral and coronary circulation. Microcirculation. 2015; 22:109–121.
Article23. Quayle JM, Dart C, Standen NB. The properties and distribution of inward rectifier potassium currents in pig coronary arterial smooth muscle. J Physiol. 1996; 494(Pt 3):715–726.
Article24. Park WS, Han J, Kim N, Ko JH, Kim SJ, Earm YE. Activation of inward rectifier K+ channels by hypoxia in rabbit coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005; 289:H2461–H2467.25. Smith PD, Brett SE, Luykenaar KD, Sandow SL, Marrelli SP, Vigmond EJ, Welsh DG. KIR channels function as electrical amplifiers in rat vascular smooth muscle. J Physiol. 2008; 586:1147–1160.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Midazolam on Outward K+ Channel Currents in Rabbit Cerebral Arterial Smooth Muscle Cells
- The Effect of Nicardipine on Capacitative Calcium Entry in Canine Pulmonary Arterial Smooth Muscle Cells
- Inhibition of voltage-dependent K⺠current in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic drug propafenone
- Two types of voltage-dependent outward potassium currents in smooth muscle cells of rabbit basilar artery
- Depolarizing Mechanisms of Acetylcholine in Gastric Smooth Muscle