Inhibition of voltage-dependent K⺠current in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic drug propafenone
- Affiliations
-
- 1Department of Physiology, Kangwon National University School of Medicine, Chuncheon 24341, Korea. parkws@kangwon.ac.kr
- KMID: 2419009
- DOI: http://doi.org/10.4196/kjpp.2018.22.5.597
Abstract
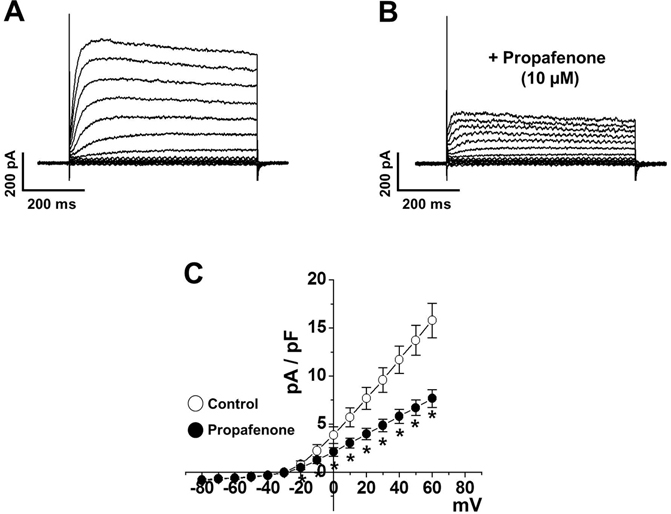
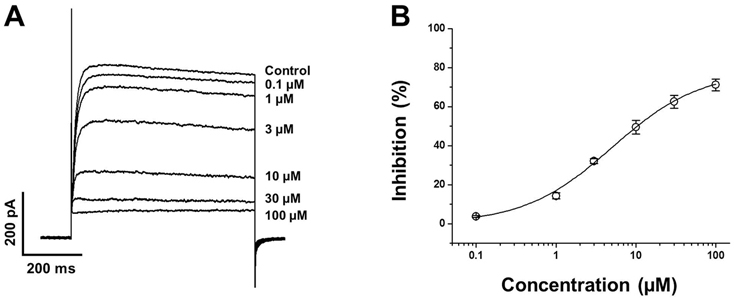
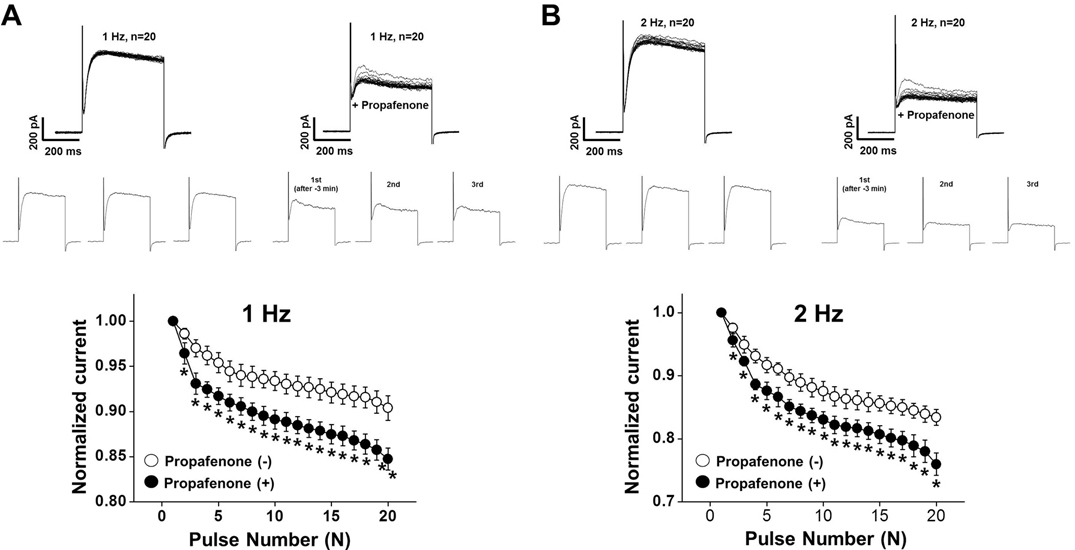
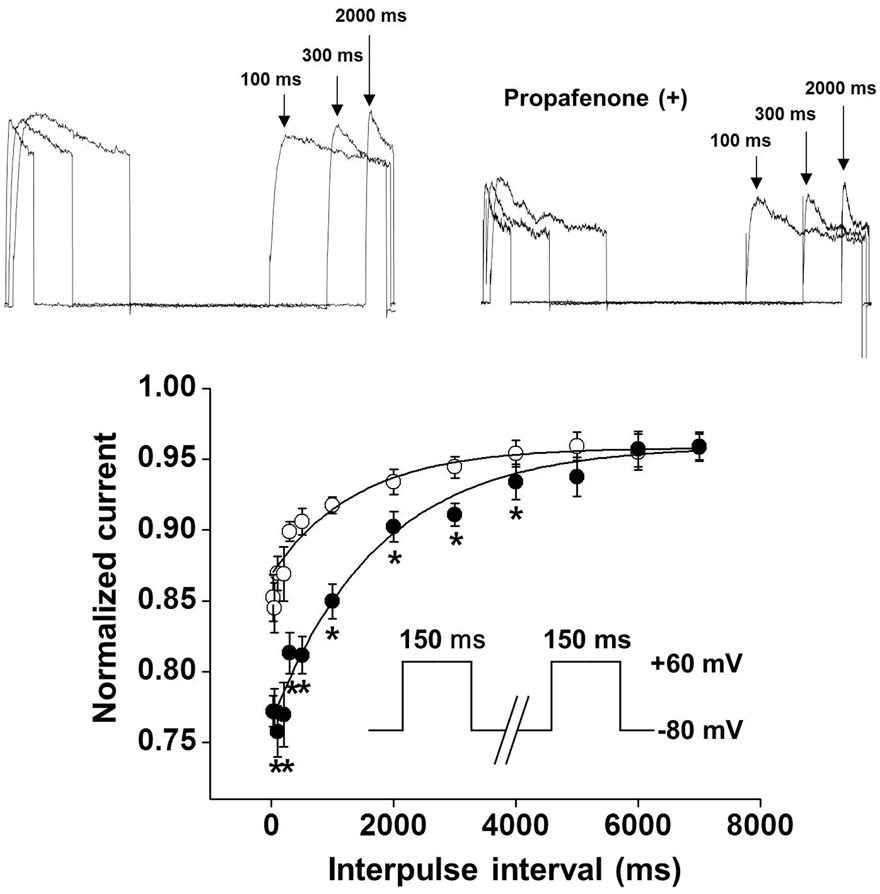
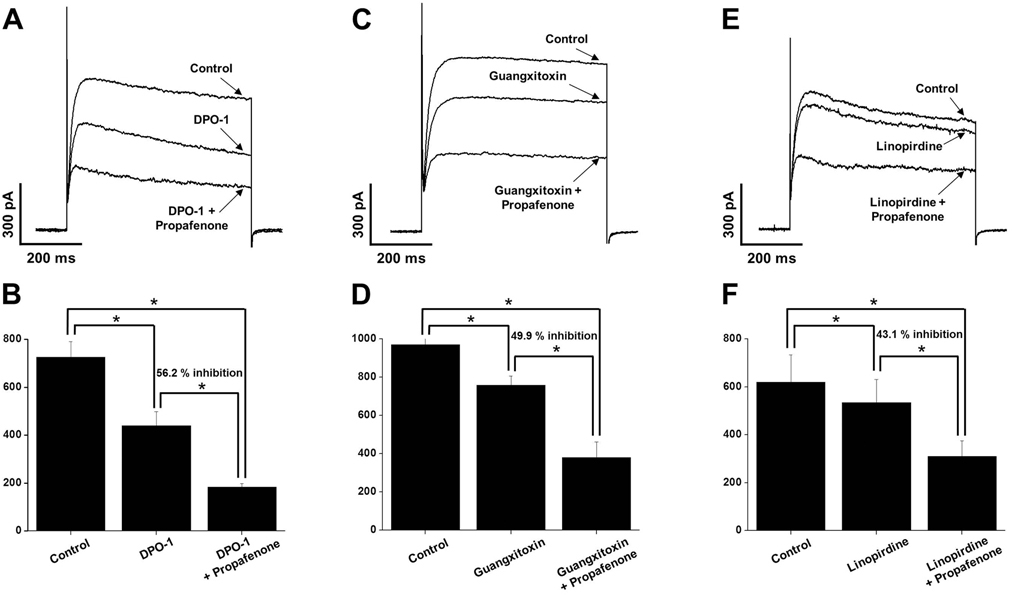
- In this study, we demonstrated the inhibitory effect of the Class Ic antiarrhythmic agent propafenone on voltage-dependent K⺠(Kv) channels using freshly isolated coronary artery smooth muscle cells from rabbits. The Kv current amplitude was progressively inhibited by propafenone in a dose-dependent manner, with an apparent ICâ‚…â‚€ value of 5.04±1.05 µM and a Hill coefficient of 0.78±0.06. The application of propafenone had no significant effect on the steady-state activation and inactivation curves, indicating that propafenone did not affect the voltage-sensitivity of Kv channels. The application of train pulses at frequencies of 1 or 2 Hz progressively increased the propafenone-induced inhibition of the Kv current. Furthermore, the inactivation recovery time constant was increased after the application of propafenone, suggesting that the inhibitory action of propafenone on Kv current is partially use-dependent. Pretreatment with Kv1.5, Kv2.1 or Kv7 inhibitor did not change the inhibitory effect of propafenone on the Kv current. Together, these results suggest that propafenone inhibits the vascular Kv channels in a dose- and use-dependent manner, regardless of Na⺠channel inhibition.
Figure
Cited by 3 articles
-
Inhibitory effects of the atypical antipsychotic, clozapine, on voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells
Minji Kang, Ryeon Heo, Seojin Park, Seo-Yeong Mun, Minju Park, Eun-Taek Han, Jin-Hee Han, Wanjoo Chun, Kwon-Soo Ha, Hongzoo Park, Won-Kyo Jung, Il-Whan Choi, Won Sun Park
Korean J Physiol Pharmacol. 2022;26(4):277-285. doi: 10.4196/kjpp.2022.26.4.277.Inhibition of voltage-dependent K+ channels by antimuscarinic drug fesoterodine in coronary arterial smooth muscle cells
Seojin Park, Minji Kang, Ryeon Heo, Seo-Yeong Mun, Minju Park, Eun-Taek Han, Jin-Hee Han, Wanjoo Chun, Hongzoo Park, Won Sun Park
Korean J Physiol Pharmacol. 2022;26(5):397-404. doi: 10.4196/kjpp.2022.26.5.397.Encainide, a class Ic anti-arrhythmic agent, blocks voltage-dependent potassium channels in coronary artery smooth muscle cells
Hongliang Li, Yue Zhou, Yongqi Yang, Yiwen Zha, Bingqian Ye, Seo-Yeong Mun, Wenwen Zhuang, Jingyan Liang, Won Sun Park
Korean J Physiol Pharmacol. 2023;27(4):399-406. doi: 10.4196/kjpp.2023.27.4.399.
Reference
-
1. Kishore AG, Camm AJ. Guidelines for the use of propafenone in treating supraventricular arrhythmias. Drugs. 1995; 50:250–262.
Article2. Benz I, Kohlhardt M. Responsiveness of cardiac Na+ channels to antiarrhythmic drugs: the role of inactivation. J Membr Biol. 1991; 122:267–278.3. Delgado C, Tamargo J, Henzel D, Lorente P. Effects of propafenone on calcium current in guinea-pig ventricular myocytes. Br J Pharmacol. 1993; 108:721–727.
Article4. Duan D, Fermini B, Nattel S. Potassium channel blocking properties of propafenone in rabbit atrial myocytes. J Pharmacol Exp Ther. 1993; 264:1113–1123.5. Delpón E, Valenzuela C, Pérez O, Casis O, Tamargo J. Propafenone preferentially blocks the rapidly activating component of delayed rectifier K+ current in guinea pig ventricular myocytes. Voltage-independent and time-dependent block of the slowly activating component. Circ Res. 1995; 76:223–235.6. Cogolludo AL, Pérez-Vizcaíno F, López-López G, Ibarra M, Zaragozá-Arnáez F, Tamargo J. Propafenone modulates potassium channel activities of vascular smooth muscle from rat portal veins. J Pharmacol Exp Ther. 2001; 299:801–810.7. Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995; 268:C799–C822.
Article8. Leblanc N, Wan X, Leung PM. Physiological role of Ca2+-activated and voltage-dependent K+ currents in rabbit coronary myocytes. Am J Physiol. 1994; 266:C1523–C1537.9. Cook NS. Effect of some potassium channel blockers on contractile responses of the rabbit aorta. J Cardiovasc Pharmacol. 1989; 13:299–306.
Article10. Hara Y, Kitamura K, Kuriyama H. Actions of 4-aminopyridine on vascular smooth muscle tissues of the guinea-pig. Br J Pharmacol. 1980; 68:99–106.
Article11. Uchida Y, Nakamura F, Tomaru T, Sumino S, Kato A, Sugimoto T. Phasic contractions of canine and human coronary arteries induced by potassium channel blockers. Jpn Heart J. 1986; 27:727–740.
Article12. Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008; 44:65–81.
Article13. Bratz IN, Dick GM, Partridge LD, Kanagy NL. Reduced molecular expression of K+ channel proteins in vascular smooth muscle from rats made hypertensive with N{omega}-nitro-L-arginine. Am J Physiol Heart Circ Physiol. 2005; 289:H1277–H1283.14. Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, Van-Raay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996; 12:17–23.
Article15. Snyders DJ, Yeola SW. Determinants of antiarrhythmic drug action. Electrostatic and hydrophobic components of block of the human cardiac hKv1.5 channel. Circ Res. 1995; 77:575–583.16. Lee HA, Hyun SA, Park SG, Kim KS, Kim SJ. Comparison of electrophysiological effects of calcium channel blockers on cardiac repolarization. Korean J Physiol Pharmacol. 2016; 20:119–127.
Article17. Kuriyama H, Kitamura K, Nabata H. Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissues. Pharmacol Rev. 1995; 47:387–573.18. Hii JT, Wyse DG, Gillis AM, Cohen JM, Mitchell LB. Propafenone-induced torsade de pointes: cross-reactivity with quinidine. Pacing Clin Electrophysiol. 1991; 14:1568–1570.
Article19. Rehnqvist N, Ericsson CG, Eriksson S, Olsson G, Svensson G. Comparative investigation of the antiarrhythmic effect of propafenone (Rytmonorm) and lidocaine in patients with ventricular arrhythmias during acute myocardial infarction. Acta Med Scand. 1984; 216:525–530.
Article20. Slawsky MT, Castle NA. K+ channel blocking actions of flecainide compared with those of propafenone and quinidine in adult rat ventricular myocytes. J Pharmacol Exp Ther. 1994; 269:66–74.21. Hoppe UC, Beuckelmann DJ. Modulation of the hyperpolarization-activated inward current (If) by antiarrhythmic agents in isolated human atrial myocytes. Naunyn Schmiedebergs Arch Pharmacol. 1998; 358:635–640.
Article22. Kim HS, Li H, Kim HW, Shin SE, Seo MS, An JR, Ha KS, Han ET, Hong SH, Choi IW, Choi G, Lee DS, Park WS. Escitalopram, a selective serotonin reuptake inhibitor, inhibits voltage-dependent K+ channels in coronary arterial smooth muscle cells. Korean J Physiol Pharmacol. 2017; 21:415–421.23. Shin SE, Li H, Kim HS, Kim HW, Seo MS, Ha KS, Han ET, Hong SH, Firth AL, Choi IW, Bae YM, Park WS. Nortriptyline, a tricyclic antidepressant, inhibits voltage-dependent K+ channels in coronary arterial smooth muscle cells. Korean J Physiol Pharmacol. 2017; 21:225–232.24. Li H, Kim HS, Kim HW, Shin SE, Jung WK, Ha KS, Han ET, Hong SH, Firth AL, Bae YM, Choi IW, Park WS. The class III anti-arrhythmic agent, amiodarone, inhibits voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol. 2016; 389:713–721.25. Xu C, Lu Y, Tang G, Wang R. Expression of voltage-dependent K+ channel genes in mesenteric artery smooth muscle cells. Am J Physiol. 1999; 277:G1055–G1063.26. Jepps TA, Olesen SP, Greenwood IA. One man's side effect is another man's therapeutic opportunity: targeting Kv7 channels in smooth muscle disorders. Br J Pharmacol. 2013; 168:19–27.
Article27. Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. Molecular composition of 4-aminopyridine-sensitive voltage-gated K+ channels of vascular smooth muscle. Circ Res. 2001; 89:1030–1037.28. Li Q, Zhang R, Lü CL, Liu Y, Wang Z, Zhu DL. The role of subtypes of voltage-gated K+ channels in pulmonary vasoconstriction induced by 15-hydroeicosatetraenoic acid. Yao Xue Xue Bao. 2006; 41:412–417.29. Cox RH, Fromme S. Functional expression profile of voltage-gated K+ channel subunits in rat small mesenteric arteries. Cell Biochem Biophys. 2016; 74:263–276.30. Stott JB, Povstyan OV, Carr G, Barrese V, Greenwood IA. G-protein βγ subunits are positive regulators of Kv7.4 and native vascular Kv7 channel activity. Proc Natl Acad Sci U S A. 2015; 112:6497–6502.
Article31. Kang KW, Shim J, Ahn J, Lee DI, Kim J, Joung B, Choi KJ. 2018 Korean heart rhythm society guidelines for antiarrhythmic drug therapy in non-valvular atrial fibrillation. Korean J Med. 2018; 93:140–152.
Article32. Siddoway LA, Roden DM, Woosley RL. Clinical pharmacology of propafenone: pharmacokinetics, metabolism and concentration-response relations. Am J Cardiol. 1984; 54:9D–12D.
Article33. Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990; 259:C3–C18.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Midazolam on Outward K+ Channel Currents in Rabbit Cerebral Arterial Smooth Muscle Cells
- Escitalopram, a selective serotonin reuptake inhibitor, inhibits voltage-dependent Kâ» channels in coronary arterial smooth muscle cells
- Effect of PKC-dependent Change of K+ Current Activity on Histamine-induced Contraction of Rabbit Coronary Artery
- Encainide, a class Ic anti-arrhythmic agent, blocks voltage-dependent potassium channels in coronary artery smooth muscle cells
- Nortriptyline, a tricyclic antidepressant, inhibits voltage-dependent K+ channels in coronary arterial smooth muscle cells