Korean J Physiol Pharmacol.
2023 Jul;27(4):399-406. 10.4196/kjpp.2023.27.4.399.
Encainide, a class Ic anti-arrhythmic agent, blocks voltage-dependent potassium channels in coronary artery smooth muscle cells
- Affiliations
-
- 1Institute of Translational Medicine, Medical College, Yangzhou University, Yangzhou 225001, China
- 2Laboratory of Integrated Traditional Chinese and Western Medicine for Prevention and Treatment for Senile Diseases, Yangzhou University, Yangzhou 225001, China
- 3Department of Physiology, Kangwon National University School of Medicine, Chuncheon 24341, Korea
- KMID: 2544141
- DOI: http://doi.org/10.4196/kjpp.2023.27.4.399
Abstract
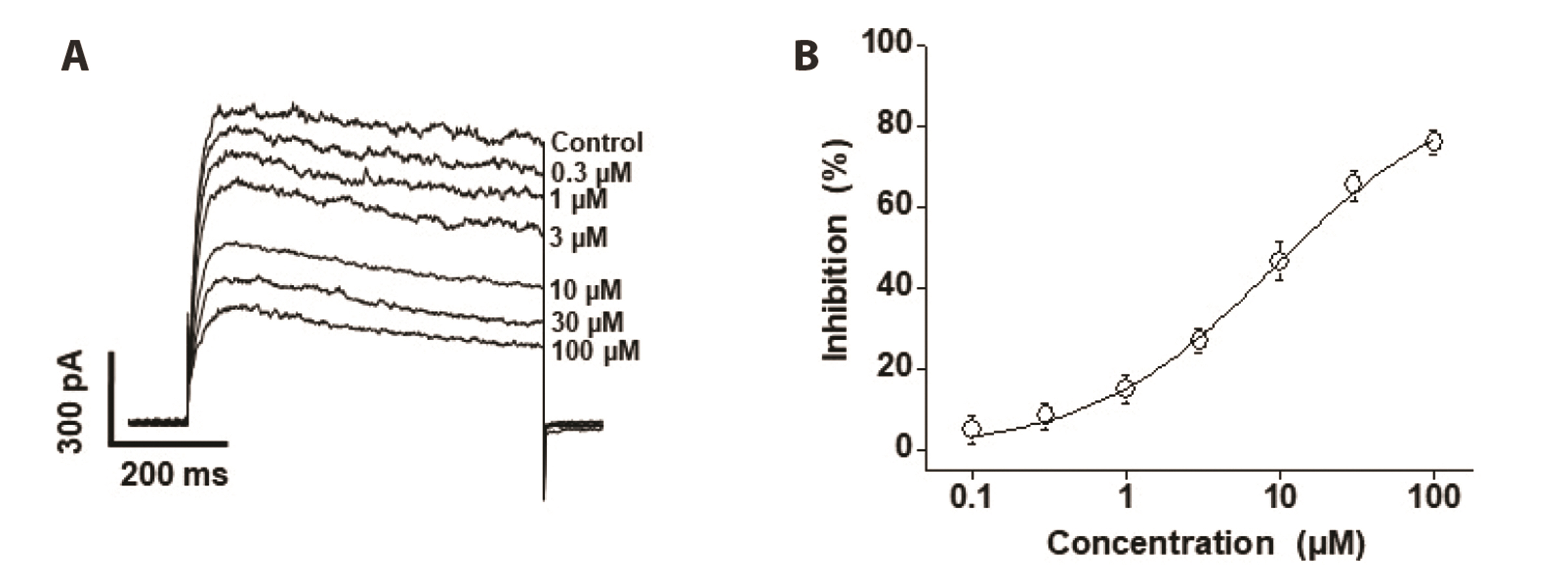
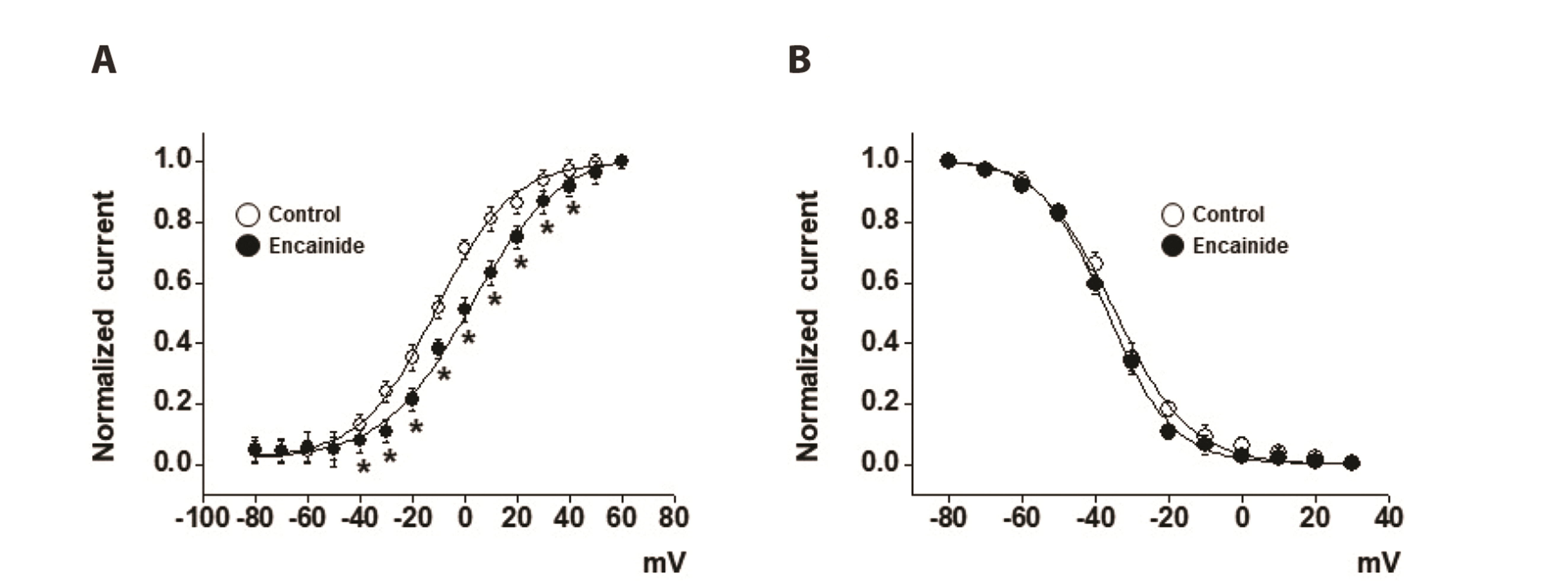
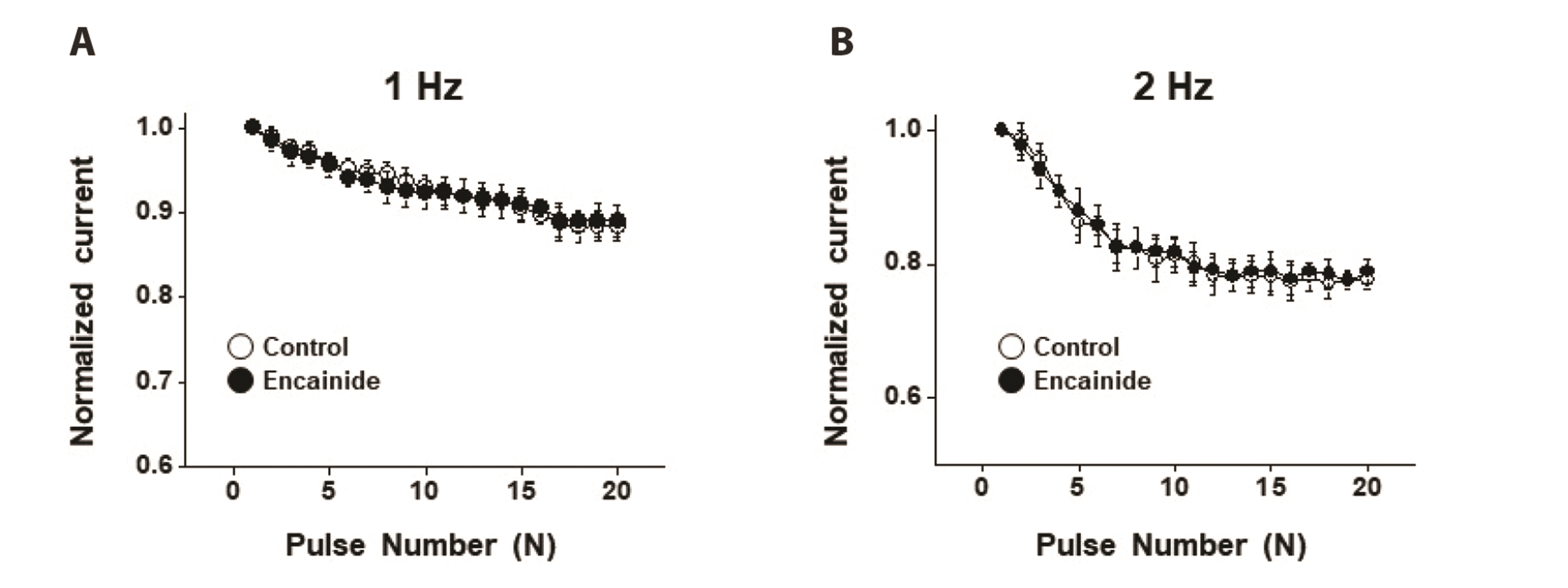
- Voltage-dependent K + (Kv) channels are widely expressed on vascular smooth muscle cells and regulate vascular tone. Here, we explored the inhibitory effect of encainide, a class Ic anti-arrhythmic agent, on Kv channels of vascular smooth muscle from rabbit coronary arteries. Encainide inhibited Kv channels in a concentration-dependent manner with an IC50 value of 8.91 ± 1.75 μM and Hill coefficient of 0.72 ± 0.06. The application of encainide shifted the activation curve toward a more positive potential without modifying the inactivation curve, suggesting that encainide inhibited Kv channels by altering the gating property of channel activation. The inhibition by encainide was not significantly affected by train pulses (1 and 2 Hz), indicating that the inhibition is not use (state)-dependent. The inhibitory effect of encainide was reduced by pretreatment with the Kv1.5 subtype inhibitor. However, pretreatment with the Kv2.1 subtype inhibitor did not alter the inhibitory effects of encainide on Kv currents. Based on these results, encainide inhibits vascular Kv channels in a concentration-dependent and use (state)-independent manner by altering the voltage sensor of the channels. Furthermore, Kv1.5 is the main Kv subtype involved in the effect of encainide.
Keyword
Figure
Reference
-
1. Goette A, Lendeckel U. 2021; Atrial cardiomyopathy: pathophysiology and clinical consequences. Cells. 10:2605. DOI: 10.3390/cells10102605. PMID: 34685585. PMCID: PMC8533786. PMID: efe620b81afd4d7e9187c6b34b055d6b.
Article2. Sagris M, Vardas EP, Theofilis P, Antonopoulos AS, Oikonomou E, Tousoulis D. 2021; Atrial fibrillation: pathogenesis, predisposing factors, and genetics. Int J Mol Sci. 23:6. DOI: 10.3390/ijms23010006. PMID: 35008432. PMCID: PMC8744894. PMID: fd0c330177d2492aa2d755a92ec15a67.
Article3. Black N, D'Souza A, Wang Y, Piggins H, Dobrzynski H, Morris G, Boyett MR. 2019; Circadian rhythm of cardiac electrophysiology, arrhythmogenesis, and the underlying mechanisms. Heart Rhythm. 16:298–307. DOI: 10.1016/j.hrthm.2018.08.026. PMID: 30170229. PMCID: PMC6520649.
Article4. Schmitt N, Grunnet M, Olesen SP. 2014; Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol Rev. 94:609–653. DOI: 10.1152/physrev.00022.2013. PMID: 24692356.
Article5. Zhang Q, Chen J, Qin Y, Wang J, Zhou L. 2018; Mutations in voltage-gated L-type calcium channel: implications in cardiac arrhythmia. Channels (Austin). 12:201–218. DOI: 10.1080/19336950.2018.1499368. PMID: 30027834. PMCID: PMC6104696.
Article6. Vaughan Williams EM. 1975; Classification of antidysrhythmic drugs. Pharmacol Ther B. 1:115–138. DOI: 10.1016/0306-039X(75)90019-7. PMID: 772700.
Article7. Antonaccio MJ, Gomoll AW, Byrne JE. 1989; Encainide. Cardiovasc Drugs Ther. 3:691–710. DOI: 10.1007/BF01857621. PMID: 2518680.
Article8. Lüderitz B, Mletzko R, Jung W, Manz M. 1991; Combination of antiarrhythmic drugs. J Cardiovasc Pharmacol. 17 Suppl 6:S48–S52. DOI: 10.1097/00005344-199100176-00011.9. Soyka LF. 1986; Safety of encainide for the treatment of ventricular arrhythmias. Am J Cardiol. 58:96C–103C. DOI: 10.1016/0002-9149(86)90111-6. PMID: 3092626.
Article10. Li H, Zhuang W, Xiong T, Park WS, Zhang S, Zha Y, Yao J, Wang F, Yang Y, Chen Y, Cai L, Ling L, Yu D, Liang J. 2022; Nrf2 deficiency attenuates atherosclerosis by reducing LOX-1-mediated proliferation and migration of vascular smooth muscle cells. Atherosclerosis. 347:1–16. DOI: 10.1016/j.atherosclerosis.2022.02.025. PMID: 35299056.
Article11. Shi J, Yang Y, Cheng A, Xu G, He F. 2020; Metabolism of vascular smooth muscle cells in vascular diseases. Am J Physiol Heart Circ Physiol. 319:H613–H631. DOI: 10.1152/ajpheart.00220.2020. PMID: 32762559.
Article12. Zhang L, Wang Y, Wu G, Rao L, Wei Y, Yue H, Yuan T, Yang P, Xiong F, Zhang S, Zhou Q, Chen Z, Li J, Mo BW, Zhang H, Xiong W, Wang CY. 2020; Blockade of JAK2 protects mice against hypoxia-induced pulmonary arterial hypertension by repressing pulmonary arterial smooth muscle cell proliferation. Cell Prolif. 53:e12742. DOI: 10.1111/cpr.12742. PMID: 31943454. PMCID: PMC7046303.
Article13. Tykocki NR, Boerman EM, Jackson WF. 2017; Smooth muscle ion channels and regulation of vascular tone in resistance arteries and arterioles. Compr Physiol. 7:485–581. DOI: 10.1002/cphy.c160011. PMID: 28333380. PMCID: PMC5575875.
Article14. Dogan MF, Yildiz O, Arslan SO, Ulusoy KG. 2019; Potassium channels in vascular smooth muscle: a pathophysiological and pharmacological perspective. Fundam Clin Pharmacol. 33:504–523. DOI: 10.1111/fcp.12461. PMID: 30851197.
Article15. Ko EA, Han J, Jung ID, Park WS. 2008; Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 44:65–81. DOI: 10.1540/jsmr.44.65. PMID: 18552454.16. Bobi J, Garabito M, Solanes N, Cidad P, Ramos-Pérez V, Ponce A, Rigol M, Freixa X, Pérez-Martínez C, Pérez de Prado A, Fernández-Vázquez F, Sabaté M, Borrós S, López-López JR, Pérez-García MT, Roqué M. 2020; Kv1.3 blockade inhibits proliferation of vascular smooth muscle cells in vitro and intimal hyperplasia in vivo. Transl Res. 224:40–54. DOI: 10.1016/j.trsl.2020.06.002. PMID: 32522668.
Article17. Jackson WF. 2010; KV1.3: a new therapeutic target to control vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol. 30:1073–1074. DOI: 10.1161/ATVBAHA.110.206565. PMID: 20484702. PMCID: PMC2891047.18. An JR, Li H, Seo MS, Park WS. 2018; Inhibition of voltage-dependent K+ current in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic drug propafenone. Korean J Physiol Pharmacol. 22:597–605. DOI: 10.4196/kjpp.2018.22.5.597. PMID: 30181706. PMCID: PMC6115351.
Article19. An JR, Kim HW, Li H, Seo MS, Jung WK, Ha KS, Han ET, Hong SH, Firth AL, Choi IW, Park WS. 2018; Inhibition of the voltage-dependent K+ current by the class Ic antiarrhythmic drug flecainide in rabbit coronary arterial smooth muscle cells. Clin Exp Pharmacol Physiol. 45:1286–1292. DOI: 10.1111/1440-1681.13015. PMID: 30028903.
Article20. Li H, Zhuang W, Seo MS, An JR, Yang Y, Zha Y, Liang J, Xu ZX, Park WS. 2021; Inhibition of voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic agent lorcainide. Eur J Pharmacol. 904:174158. DOI: 10.1016/j.ejphar.2021.174158. PMID: 33971179.21. Hasan R, Jaggar JH. 2018; KV channel trafficking and control of vascular tone. Microcirculation. 25:10.1111/micc.12418. DOI: 10.1111/micc.12418. PMID: 28963858. PMCID: PMC5760341.22. Lu Y, Hanna ST, Tang G, Wang R. 2002; Contributions of Kv1.2, Kv1.5 and Kv2.1 subunits to the native delayed rectifier K(+) current in rat mesenteric artery smooth muscle cells. Life Sci. 71:1465–1473. DOI: 10.1016/S0024-3205(02)01922-7. PMID: 12127166.
Article23. Jaillon P. 1990; Pharmacokinetics and metabolism of encainide. Cardiovasc Drugs Ther. 4 Suppl 3:561–565. DOI: 10.1007/BF00357031. PMID: 2125833.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of voltage-dependent K⺠current in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic drug propafenone
- Effects of Fluoxetine on Potassium Channels in Cat Gastric Smooth Muscle Cells
- The Effect Erythrocyte lysate on Intracellular Calcium Concentration and the Potassium Channel in Cerebral Vasular Smooth Muscle Cells
- Effects of Propofol in Voltage-dependent Potassium Channels in Human Neurl Stem Cells
- Physiological Roles and Properties of Ion Channels in Corporal Smooth Muscle