Intest Res.
2019 Oct;17(4):476-485. 10.5217/ir.2019.00027.
Tacrolimus for ulcerative colitis in children: a multicenter survey in Japan
- Affiliations
-
- 1Department of Pediatrics and Child Health, Kurume University School of Medicine, Kurume, Japan. yanagi_tadahiro@med.kurume-u.ac.jp
- 2Members of the Japanese Society for Pediatric Inflammatory Bowel Disease Working Group, Japan.
- 3Clinical Research Support Office, Aso Iizuka Hospital, Iizuka, Japan.
- 4PAL Children’s Clinic, Isesaki, Japan.
- 5Department of Pediatrics, Osaka General Medical Center, Osaka, Japan.
- 6Inflammatory Bowel Center, Yokohama City University Medical Center, Yokohama, Japan.
- 7Department of Pediatrics, Gunma University Graduate School of Medicine, Maebashi, Japan.
- 8Department of Pediatric Gastroenterology, Nutrition and Endocrinology, Osaka Women’s and Children’s Hospital, Osaka, Japan.
- 9Division of Gastroenterology, National Center for Child Health and Development, Tokyo, Japan.
- 10Department of Pediatrics, Osaka Medical College, Osaka, Japan.
- 11Department of Pediatrics, Tokyo Women’s Medical University Hospital, Tokyo, Japan.
- 12Department of Gastrointestinal and Pediatric Surgery, Mie University Graduate School of Medicine, Tsu, Japan.
- 13Department of Pediatrics and Adolescent Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan.
- KMID: 2465813
- DOI: http://doi.org/10.5217/ir.2019.00027
Abstract
- BACKGROUND/AIMS
Tacrolimus is effective for refractory ulcerative colitis in adults, while data for children is sparse. We aimed to evaluate the effectiveness and safety of tacrolimus for induction and maintenance therapy in Japanese children with ulcerative colitis.
METHODS
We retrospectively reviewed the multicenter survey data of 67 patients with ulcerative colitis aged < 17 years treated with tacrolimus between 2000 and 2012. Patients' characteristics, disease activity, Pediatric Ulcerative Colitis Activity Index (PUCAI) score, initial oral tacrolimus dose, short-term (2-week) and long-term (1-year) outcomes, steroid-sparing effects, and adverse events were evaluated. Clinical remission was defined as a PUCAI score < 10; treatment response was defined as a PUCAI score reduction of ≥ 20 points compared with baseline.
RESULTS
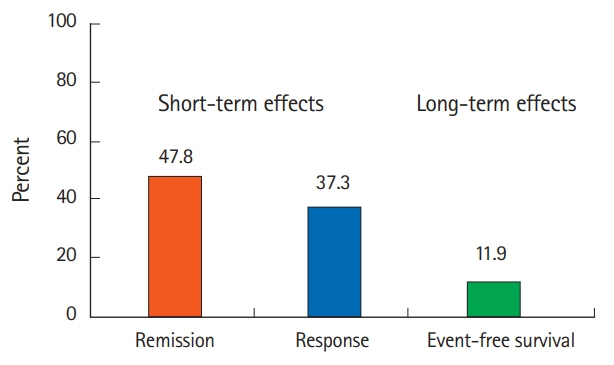
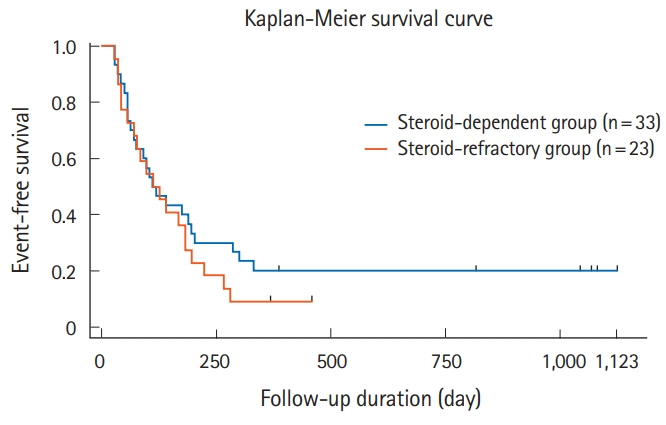
Patients included 35 boys and 32 girls (median [interquartile range] at admission: 13 [11-15] years). Thirty-nine patients were steroid-dependent and 26 were steroidrefractory; 20 had severe colitis and 43 had moderate colitis. The initial tacrolimus dose was 0.09 mg/kg/day (range, 0.05-0.12 mg/kg/day). The short-term clinical remission rate was 47.8%, and the clinical response rate was 37.3%. The mean prednisolone dose was reduced from 19.2 mg/day at tacrolimus initiation to 5.7 mg/day at week 8 (P< 0.001). The adverse event rate was 53.7%; 6 patients required discontinuation of tacrolimus therapy.
CONCLUSIONS
Tacrolimus was a safe and effective second-line induction therapy for steroid-dependent and steroid-refractory ulcerative colitis in Japanese children.
MeSH Terms
Figure
Cited by 1 articles
-
Does cytomegalovirus load predict the outcome of acute severe ulcerative colitis?
You Sun Kim
Intest Res. 2021;19(4):357-359. doi: 10.5217/ir.2021.00120.
Reference
-
1. Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008; 135:1114–1122.2. Ishige T, Tomomasa T, Takebayashi T, et al. Inflammatory bowel disease in children: epidemiological analysis of the nationwide IBD registry in Japan. J Gastroenterol. 2010; 45:911–917.
Article3. Turner D, Levine A, Escher JC, et al. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr. 2012; 55:340–361.4. Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of paediatric ulcerative colitis, part 2: acute severe colitis-an evidence-based consensus guideline from the European Crohn’s and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018; 67:292–310.5. Navas-López VM, Blasco Alonso J, Serrano Nieto MJ, Girón Fernández-Crehuet F, Argos Rodriguez MD, Sierra Salinas C. Oral tacrolimus for pediatric steroid-resistant ulcerative colitis. J Crohns Colitis. 2014; 8:64–69.6. Turner D, Griffiths AM. Acute severe ulcerative colitis in children: a systematic review. Inflamm Bowel Dis. 2011; 17:440–449.7. Kino T, Hatanaka H, Miyata S, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot (Tokyo). 1987; 40:1256–1265.8. Kelly PA, Burckart GJ, Venkataramanan R. Tacrolimus: a new immunosuppressive agent. Am J Health Syst Pharm. 1995; 52:1521–1535.9. Kino T, Hatanaka H, Hashimoto M, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot (Tokyo). 1987; 40:1249–1255.
Article10. Ogata H, Matsui T, Nakamura M, et al. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut. 2006; 55:1255–1262.11. Komaki Y, Komaki F, Ido A, Sakuraba A. Efficacy and safety of tacrolimus therapy for active ulcerative colitis; a systematic review and meta-analysis. J Crohns Colitis. 2016; 10:484–494.12. Matsuoka K, Hibi T. Treatment guidelines in inflammatory bowel disease: the Japanese perspectives. Dig Dis. 2013; 31:363–367.13. IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis-the Porto criteria. J Pediatr Gastroenterol Nutr. 2005; 41:1–7.14. Marion JF, Rubin PH, Present DH. Differential diagnosis of chronic ulcerative colitis and Crohn’s disease. In : Kirsner JB, editor. Inflammatory bowel disease. 5th ed. Philadelphia: WB Saunders;2000. p. 315–325.15. Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a Pediatric Ulcerative Colitis Activity Index: a prospective multicenter study. Gastroenterology. 2007; 133:423–432.16. Romano C, Syed S, Valenti S, Kugathasan S. Management of acute severe colitis in children with ulcerative colitis in the biologics era. Pediatrics. 2016; 137:e20151184.17. Turner D, Walsh CM, Benchimol EI, et al. Severe paediatric ulcerative colitis: incidence, outcomes and optimal timing for second-line therapy. Gut. 2008; 57:331–338.18. Turner D, Mack D, Leleiko N, et al. Severe pediatric ulcerative colitis: a prospective multicenter study of outcomes and predictors of response. Gastroenterology. 2010; 138:2282–2291.19. Bousvaros A, Wang A, Leichtner AM. Tacrolimus (FK-506) treatment of fulminant colitis in a child. J Pediatr Gastroenterol Nutr. 1996; 23:329–333.20. Bousvaros A, Kirschner BS, Werlin SL, et al. Oral tacrolimus treatment of severe colitis in children. J Pediatr. 2000; 137:794–799.
Article21. Ziring DA, Wu SS, Mow WS, Martín MG, Mehra M, Ament ME. Oral tacrolimus for steroid-dependent and steroid-resistant ulcerative colitis in children. J Pediatr Gastroenterol Nutr. 2007; 45:306–311.22. Watson S, Pensabene L, Mitchell P, Bousvaros A. Outcomes and adverse events in children and young adults undergoing tacrolimus therapy for steroid-refractory colitis. Inflamm Bowel Dis. 2011; 17:22–29.23. Hamel B, Wu M, Hamel EO, Bass DM, Park KT. Outcome of tacrolimus and vedolizumab after corticosteroid and anti-TNF failure in paediatric severe colitis. BMJ Open Gastroenterol. 2018; 5:e000195.24. Hosoi K, Arai K, Matsuoka K, et al. Prolonged tacrolimus for pediatric gastrointestinal disorder: double-edged sword? Pediatr Int. 2017; 59:588–592.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tacrolimus in pediatric ulcerative colitis: does it have a role?
- Fecal Microbiota Transplantation to Patients with Refractory Very Early Onset Ulcerative Colitis
- Pustular Pyoderma Gangrenosum Associated with Ulcerative Colitis
- Tacrolimus for the Treatment of Ulcerative Colitis
- Individualized treatment based on CYP3A5 single-nucleotide polymorphisms with tacrolimus in ulcerative colitis