Intest Res.
2019 Apr;17(2):218-226. 10.5217/ir.2018.00117.
Individualized treatment based on CYP3A5 single-nucleotide polymorphisms with tacrolimus in ulcerative colitis
- Affiliations
-
- 1Center for Advanced IBD Research and Treatment, Department of Research, Kitasato University Kitasato Institute Hospital, Tokyo, Japan. kobataku@insti.kitasato-u.ac.jp
- 2Department of Gastroenterology and Hepatology, Kitasato University Kitasato Institute Hospital, Tokyo, Japan.
- 3Department of Rheumatology, Kitasato University Kitasato Institute Hospital, Tokyo, Japan.
- 4Department of Pharmacy, Kitasato University Kitasato Institute Hospital, Tokyo, Japan.
- 5Biomedical Laboratory, Department of Research, Kitasato University Kitasato Institute Hospital, Tokyo, Japan.
- KMID: 2449963
- DOI: http://doi.org/10.5217/ir.2018.00117
Abstract
- BACKGROUND/AIMS
The pharmacokinetics of tacrolimus (TAC) is known to be largely influenced by single-nucleotide polymorphisms (SNPs) in CYP3A5. Patients starting TAC require careful dose adjustment, owing to the wide range of optimal dosages, depending on their CYP3A5 expression status. Here, we evaluated whether individualization of TAC dosages based on CYP3A5 SNPs would improve its therapeutic efficacy in ulcerative colitis.
METHODS
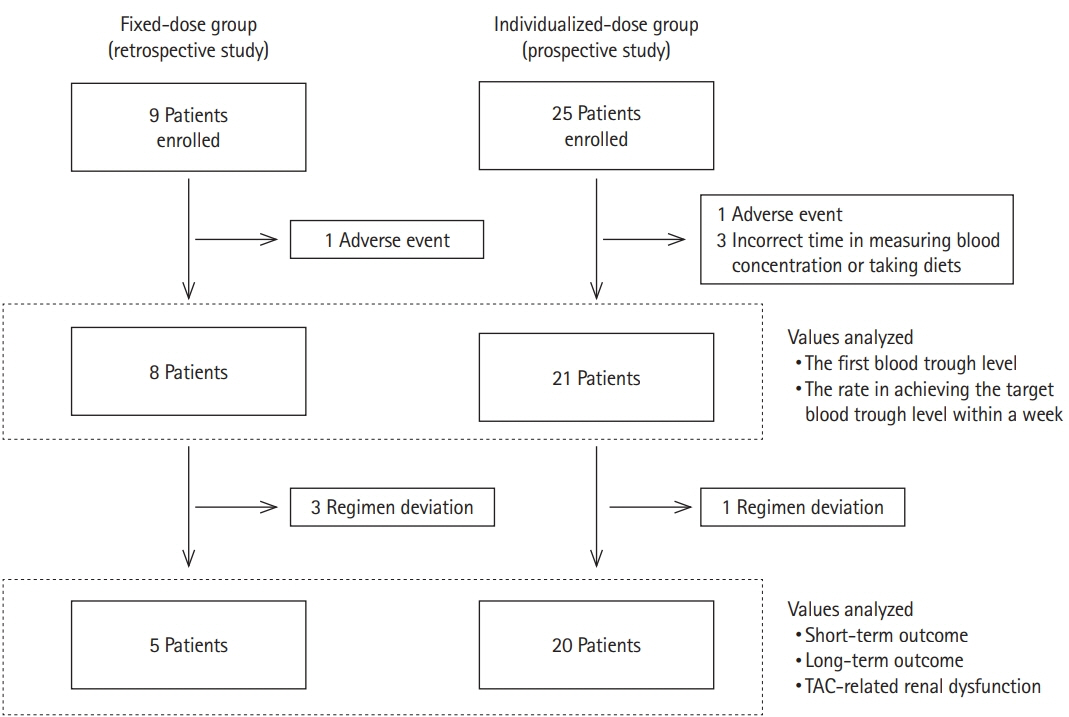
Twenty-one patients were prospectively treated, with their initial dosage adjusted according to their CYP3A5 status (0.1, 0.15, and 0.2 mg/kg/day for CYP3A5*3/*3, CYP3A5*1/*3, and CYP3A5*1/*1, respectively). Their clinical outcomes were compared with those of patients treated with a fixed dose (0.1 mg/kg/day).
RESULTS
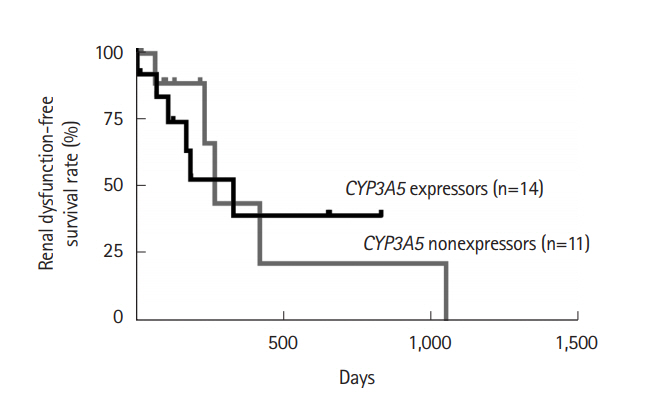
The first blood trough level of CYP3A5 expressors, CYP3A5*1/*3 or CYP3A5*1/*1, and the overall rate in achieving the target blood trough level within a week in the individualized-dose group were significantly higher than those in the fixed-dose group (5.15±2.33 ng/mL vs. 9.63±0.79 ng/mL, P=0.035 and 12.5% vs. 66.7%, P=0.01). The remission rate at 2 weeks in the expressors was as high as that in the nonexpressors, CYP3A5*3/*3, in the individualized-dose group.
CONCLUSIONS
Individualized TAC treatment is effective against ulcerative colitis regardless of the CYP3A5 genotype.
MeSH Terms
Figure
Reference
-
1. Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007; 369:1627–1640.
Article2. Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis. 2010; 4:431–437.
Article3. Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007; 5:103–110.
Article4. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.
Article5. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017; 11:769–784.
Article6. Komaki Y, Komaki F, Ido A, Sakuraba A. Efficacy and safety of tacrolimus therapy for active ulcerative colitis; a systematic review and meta-analysis. J Crohns Colitis. 2016; 10:484–494.
Article7. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992; 13:136–142.
Article8. Kelly PA, Burckart GJ, Venkataramanan R. Tacrolimus: a new immunosuppressive agent. Am J Health Syst Pharm. 1995; 52:1521–1535.
Article9. Ogata H, Kato J, Hirai F, et al. Double-blind, placebo-controlled trial of oral tacrolimus (FK506) in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflamm Bowel Dis. 2012; 18:803–808.
Article10. Ogata H, Matsui T, Nakamura M, et al. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut. 2006; 55:1255–1262.
Article11. Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009; 4:481–508.
Article12. Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001; 27:383–391.
Article13. Schwab M, Eichelbaum M, Fromm MF. Genetic polymorphisms of the human MDR1 drug transporter. Annu Rev Pharmacol Toxicol. 2003; 43:285–307.14. Hirai F, Takatsu N, Yano Y, et al. Impact of CYP3A5 genetic polymorphisms on the pharmacokinetics and short-term remission in patients with ulcerative colitis treated with tacrolimus. J Gastroenterol Hepatol. 2014; 29:60–66.
Article15. Herrlinger KR, Koc H, Winter S, et al. ABCB1 single-nucleotide polymorphisms determine tacrolimus response in patients with ulcerative colitis. Clin Pharmacol Ther. 2011; 89:422–428.
Article16. Onodera M, Endo K, Kakuta Y, et al. ATP-binding cassette subfamily B member 1 1236C/T polymorphism significantly affects the therapeutic outcome of tacrolimus in patients with refractory ulcerative colitis. J Gastroenterol Hepatol. 2017; 32:1562–1569.
Article17. Barbarino JM, Staatz CE, Venkataramanan R, Klein TE, Altman RB. PharmGKB summary: cyclosporine and tacrolimus pathways. Pharmacogenet Genomics. 2013; 23:563–585.18. Provenzani A, Notarbartolo M, Labbozzetta M, et al. Influence of CYP3A5 and ABCB1 gene polymorphisms and other factors on tacrolimus dosing in Caucasian liver and kidney transplant patients. Int J Mol Med. 2011; 28:1093–1102.
Article19. Satoh S, Saito M, Inoue T, et al. CYP3A5*1 allele associated with tacrolimus trough concentrations but not subclinical acute rejection or chronic allograft nephropathy in Japanese renal transplant recipients. Eur J Clin Pharmacol. 2009; 65:473–481.
Article20. Suzuki Y, Homma M, Doki K, Itagaki F, Kohda Y. Impact of CYP3A5 genetic polymorphism on pharmacokinetics of tacrolimus in healthy Japanese subjects. Br J Clin Pharmacol. 2008; 66:154–155.
Article21. Bannai M, Higuchi K, Akesaka T, et al. Single-nucleotide-polymorphism genotyping for whole-genome-amplified samples using automated fluorescence correlation spectroscopy. Anal Biochem. 2004; 327:215–221.
Article22. Bekersky I, Dressler D, Mekki Q. Effect of time of meal consumption on bioavailability of a single oral 5 mg tacrolimus dose. J Clin Pharmacol. 2001; 41:289–297.
Article23. Tada H, Tsuchiya N, Satoh S, et al. Impact of CYP3A5 and MDR1(ABCB1) C3435T polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplant Proc. 2005; 37:1730–1732.
Article24. Ball SE, Scatina J, Kao J, et al. Population distribution and effects on drug metabolism of a genetic variant in the 5’ promoter region of CYP3A4. Clin Pharmacol Ther. 1999; 66:288–294.
Article25. Sata F, Sapone A, Elizondo G, et al. CYP3A4 allelic variants with amino acid substitutions in exons 7 and 12: evidence for an allelic variant with altered catalytic activity. Clin Pharmacol Ther. 2000; 67:48–56.
Article26. Tsuchiya N, Satoh S, Tada H, et al. Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation. 2004; 78:1182–1187.
Article27. Masuda S, Inui K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther. 2006; 112:184–198.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tacrolimus in pediatric ulcerative colitis: does it have a role?
- Effect of cytochrome P450 3A5 polymorphism on the pharmacokinetics of tacrolimus in renal transplant recipients
- Pustular Pyoderma Gangrenosum Associated with Ulcerative Colitis
- Tacrolimus for the Treatment of Ulcerative Colitis
- Influencing Factors for Cure of Clonorchiasis by Praziquantel Therapy: Infection Burden and CYP3A5 Gene Polymorphism