Neutrophils as a Protagonist and Target in Chronic Rhinosinusitis
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. zhengliuent@hotmail.com
- KMID: 2462729
- DOI: http://doi.org/10.21053/ceo.2019.00654
Abstract
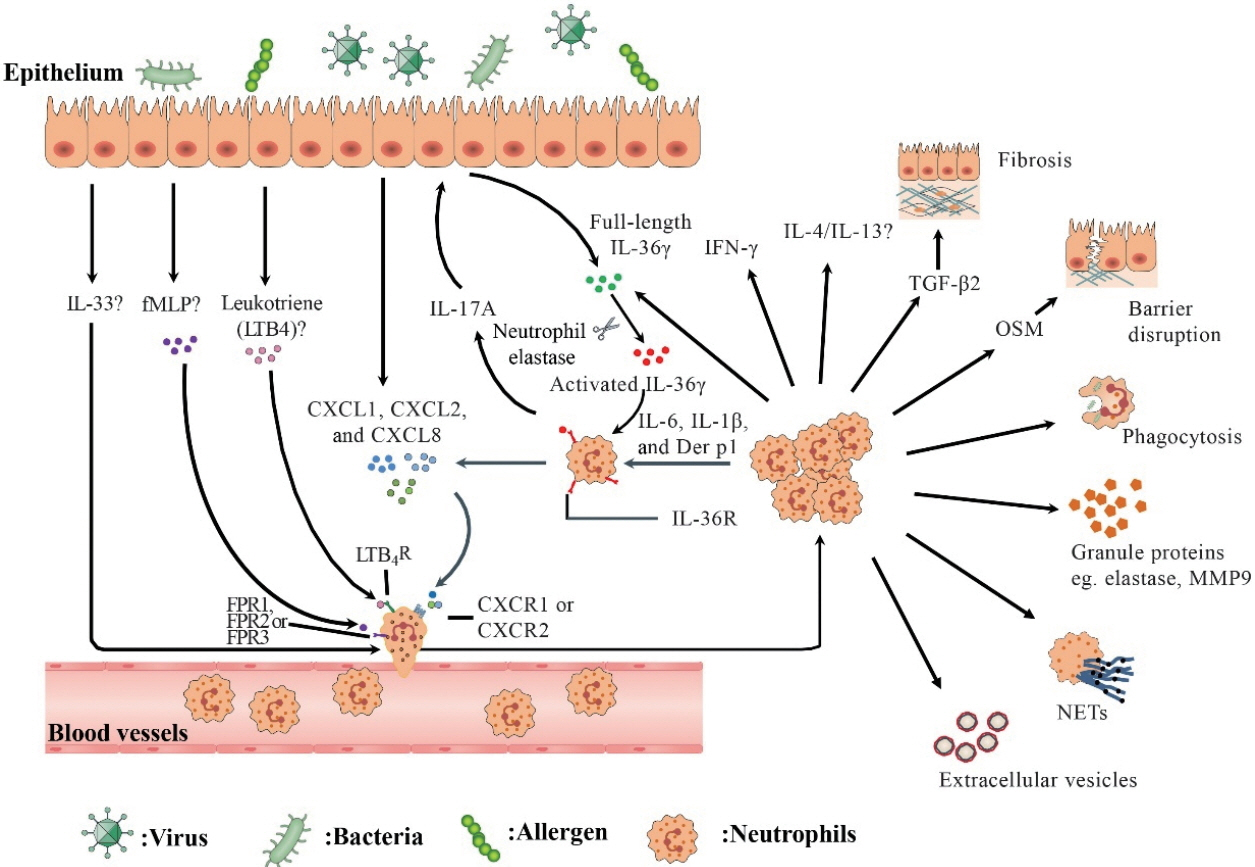
- Neutrophils have traditionally been acknowledged as the first immune cells that are recruited to inflamed tissues during acute inflammation. By contrast, their importance in the context of chronic inflammation has been studied in less depth. Neutrophils can be recruited and are largely present in the nasal mucosa of patients with chronic rhinosinusitis (CRS) both in Asians and in Caucasians. Increased infiltration of neutrophils in patients with CRS has been linked to poor corticosteroid response and disease prognosis. Meanwhile, tissue neutrophils may possess specific phenotypic features distinguishing them from resting blood counterparts and are endowed with particular functions, such as cytokines and chemokines production, thus may contribute to the pathogenesis of CRS. This review aims to summarize our current understanding of the pathophysiologic mechanisms of CRS, with a focus on the roles of neutrophils. We discuss recruitment, function, and regulation of neutrophils in CRS and outline the potential therapeutic strategies targeting neutrophils.
MeSH Terms
Figure
Cited by 5 articles
-
Chinese Society of Allergy and Chinese Society of Otorhinolaryngology-Head and Neck Surgery Guideline for Chronic Rhinosinusitis
Zheng Liu, Jianjun Chen, Lei Cheng, Huabin Li, Shixi Liu, Hongfei Lou, Jianbo Shi, Ying Sun, Dehui Wang, Chengshuo Wang, Xiangdong Wang, Yongxiang Wei, Weiping Wen, Pingchang Yang, Qintai Yang, Gehua Zhang, Yuan Zhang, Changqing Zhao, Dongdong Zhu, Li Zhu, Fenghong Chen, Yi Dong, Qingling Fu, Jingyun Li, Yanqing Li, Chengyao Liu, Feng Liu, Meiping Lu, Yifan Meng, Jichao Sha, Wenyu She, Lili Shi, Kuiji Wang, Jinmei Xue, Luoying Yang, Min Yin, Lichuan Zhang, Ming Zheng, Bing Zhou, Luo Zhang
Allergy Asthma Immunol Res. 2020;12(2):176-237. doi: 10.4168/aair.2020.12.2.176.Role of IL-17A in Chronic Rhinosinusitis With Nasal Polyp
Gwanghui Ryu, Jun-Sang Bae, Ji Hye Kim, Eun Hee Kim, Lele Lyu, Young-Jun Chung, Ji-Hun Mo
Allergy Asthma Immunol Res. 2020;12(3):507-522. doi: 10.4168/aair.2020.12.3.507.Can Neutrophils Be a Cellular Biomarker in Asian Chronic Rhinosinusitis?
Dae Woo Kim
Clin Exp Otorhinolaryngol. 2019;12(4):325-326. doi: 10.21053/ceo.2019.01452.Effect of Obstructive Sleep Apnea on Immunity in Cases of Chronic Rhinosinusitis With Nasal Polyps
Dong-Kyu Kim, Byeong Chan Lee, Ki Joon Park, Gil Myeong Son
Clin Exp Otorhinolaryngol. 2021;14(4):390-398. doi: 10.21053/ceo.2020.02250.Considerations for the Use of Biologic Agents in Patients With Chronic Rhinosinusitis With Nasal Polyposis
Do Hyun Kim, Sung Won Kim
Clin Exp Otorhinolaryngol. 2021;14(3):245-246. doi: 10.21053/ceo.2021.01249.
Reference
-
1. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012: a summary for otorhinolaryngologists. Rhinology. 2012; Mar. 50(1):1–12.
Article2. Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol. 2017; Jan. 12(1):331–57.
Article3. Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol. 2015; Dec. 136(6):1431–40.
Article4. Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016; Nov. 138(5):1344–53.5. Huvenne W, van Bruaene N, Zhang N, van Zele T, Patou J, Gevaert P, et al. Chronic rhinosinusitis with and without nasal polyps: what is the difference. Curr Allergy Asthma Rep. 2009; May. 9(3):213–20.
Article6. Wang H, Li ZY, Jiang WX, Liao B, Zhai GT, Wang N, et al. The activation and function of IL-36γ in neutrophilic inflammation in chronic rhinosinusitis. J Allergy Clin Immunol. 2018; May. 141(5):1646–58.
Article7. Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009; Sep. 124(3):478–84.
Article8. Zhang Y, Gevaert E, Lou H, Wang X, Zhang L, Bachert C, et al. Chronic rhinosinusitis in Asia. J Allergy Clin Immunol. 2017; Nov. 140(5):1230–9.
Article9. Fan Y, Chen S, Qu X, Zuo K, Li X, Huang J, et al. A lower prevalence of asthma among patients with chronic rhinosinusitis in southern China. J Allergy Clin Immunol. 2011; Feb. 127(2):520–2.e1-5.
Article10. Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008; Nov. 122(5):961–8.
Article11. Cao PP, Wang ZC, Schleimer RP, Liu Z. Pathophysiologic mechanisms of chronic rhinosinusitis and their roles in emerging disease endotypes. Ann Allergy Asthma Immunol. 2019; Jan. 122(1):33–40.
Article12. Cao PP, Zhang YN, Liao B, Ma J, Wang BF, Wang H, et al. Increased local IgE production induced by common aeroallergens and phenotypic alteration of mast cells in Chinese eosinophilic, but not noneosinophilic, chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2014; Mar. 44(5):690–700.
Article13. Payne SC, Early SB, Huyett P, Han JK, Borish L, Steinke JW. Evidence for distinct histologic profile of nasal polyps with and without eosinophilia. Laryngoscope. 2011; Oct. 121(10):2262–7.
Article14. Kim DK, Jin HR, Eun KM, Mutusamy S, Cho SH, Oh S, et al. Noneosinophilic nasal polyps shows increased epithelial proliferation and localized disease pattern in the early stage. PLoS One. 2015; Oct. 10(10):e0139945.
Article15. Shi LL, Song J, Xiong P, Cao PP, Liao B, Ma J, et al. Disease-specific T-helper cell polarizing function of lesional dendritic cells in different types of chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2014; Sep. 190(6):628–38.
Article16. Shi LL, Xiong P, Zhang L, Cao PP, Liao B, Lu X, et al. Features of airway remodeling in different types of Chinese chronic rhinosinusitis are associated with inflammation patterns. Allergy. 2013; Jan. 68(1):101–9.
Article17. Ma J, Shi LL, Deng YK, Wang H, Cao PP, Long XB, et al. CD8(+) T cells with distinct cytokine-producing features and low cytotoxic activity in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2016; Sep. 46(9):1162–75.18. Pothoven KL, Norton JE, Suh LA, Carter RG, Harris KE, Biyasheva A, et al. Neutrophils are a major source of the epithelial barrier disrupting cytokine oncostatin M in patients with mucosal airways disease. J Allergy Clin Immunol. 2016; Jun. 139(6):1966–78.e9.
Article19. Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010; Nov. 126(5):962–8.e1-6.
Article20. Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016; May. 137(5):1449–56.e4.
Article21. Wen W, Liu W, Zhang L, Bai J, Fan Y, Xia W, et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol. 2012; Jun. 129(6):1522–8.e5.
Article22. Liao B, Liu JX, Li ZY, Zhen Z, Cao PP, Yao Y, et al. Multidimensional endotypes of chronic rhinosinusitis and their association with treatment outcomes. Allergy. 2018; Jul. 73(7):1459–69.
Article23. Soehnlein O, Steffens S, Hidalgo A, Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol. 2017; Apr. 17(4):248–61.
Article24. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016; Jul. 16(7):431–46.
Article25. Ley K, Hoffman HM, Kubes P, Cassatella MA, Zychlinsky A, Hedrick CC, et al. Neutrophils: new insights and open questions. Sci Immunol. 2018; Dec. 3(30)::eaat4579.
Article26. de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016; May. 16(6):378–91.
Article27. Shiozawa A, Miwa M, Ono N, Homma H, Hirotsu M, Ikeda K. Comparative analysis of cytokine release from epithelial cell cultures of the upper airway. Rhinology. 2015; Jun. 53(2):135–41.
Article28. Shimizu S, Kouzaki H, Kato T, Tojima I, Shimizu T. HMGB1-TLR4 signaling contributes to the secretion of interleukin 6 and interleukin 8 by nasal epithelial cells. Am J Rhinol Allergy. 2016; May. 30(3):167–72.
Article29. Rammal A, Tewfik M, Rousseau S. Differences in RANTES and IL-6 levels among chronic rhinosinusitis patients with predominant gramnegative and gram-positive infection. J Otolaryngol Head Neck Surg. 2017; Jan. 46(1):7.
Article30. Ozturk AB, Bayraktar R, Gogebakan B, Mumbuc S, Bayram H. Comparison of inflammatory cytokine release from nasal epithelial cells of non-atopic non-rhinitic, allergic rhinitic and polyp subjects and effects of diesel exhaust particles in vitro. Allergol Immunopathol (Madr). 2017; Sep-Oct. 45(5):473–81.
Article31. Tsai YJ, Hao SP, Chen CL, Wu WB. Thromboxane A2 regulates CXCL1 and CXCL8 chemokine expression in the nasal mucosaderived fibroblasts of chronic rhinosinusitis patients. PLoS One. 2016; Jun. 11(6):e0158438.
Article32. Shimizu S, Tojima I, Takezawa K, Matsumoto K, Kouzaki H, Shimizu T. Thrombin and activated coagulation factor X stimulate the release of cytokines and fibronectin from nasal polyp fibroblasts via protease-activated receptors. Am J Rhinol Allergy. 2017; Jan. 31(1):13–8.
Article33. Zhai GT, Wang H, Li JX, Cao PP, Jiang WX, Song J, et al. IgD-activated mast cells induce IgE synthesis in B cells in nasal polyps. J Allergy Clin Immunol. 2018; Nov. 142(5):1489–99.e23.
Article34. Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006; Nov. 61(11):1280–9.
Article35. Kim DW, Eun KM, Roh EY, Shin S, Kim DK. Chronic rhinosinusitis without nasal polyps in Asian patients shows mixed inflammatory patterns and neutrophil-related disease severity. Mediators Inflamm. 2019; Jan. 2019:7138643.
Article36. Cho DY, Nayak JV, Bravo DT, Le W, Nguyen A, Edward JA, et al. Expression of dual oxidases and secreted cytokines in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013; May. 3(5):376–83.
Article37. Wei B, Liu F, Zhang J, Liu Y, Du J, Liu S, et al. Multivariate analysis of inflammatory endotypes in recurrent nasal polyposis in a Chinese population. Rhinology. 2018; Sep. 56(3):216–26.
Article38. Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007; 25(1):821–52.
Article39. Pridgeon C, Bugeon L, Donnelly L, Straschil U, Tudhope SJ, Fenwick P, et al. Regulation of IL-17 in chronic inflammation in the human lung. Clin Sci (Lond). 2011; Jun. 120(12):515–24.
Article40. Niu YZ, Gong GQ, Chen S, Chen JJ, Kong WJ, Wang YJ. Effects of IL-17 on expression of GRO-α and IL-8 in fibroblasts from nasal polyps. J Huazhong Univ Sci Technolog Med Sci. 2014; Aug. 34(4):591–5.
Article41. Wang H, Bai J, Ding M, Liu W, Xu R, Zhang J, et al. Interleukin-17A contributes to the expression of serum amyloid A in chronic rhinosinusitis with nasal polyps. Eur Arch Otorhinolaryngol. 2013; May. 270(6):1867–72.
Article42. Saitoh T, Kusunoki T, Yao T, Kawano K, Kojima Y, Miyahara K, et al. Role of interleukin-17A in the eosinophil accumulation and mucosal remodeling in chronic rhinosinusitis with nasal polyps associated with asthma. Int Arch Allergy Immunol. 2010; 151(1):8–16.
Article43. Makihara S, Okano M, Fujiwara T, Kariya S, Noda Y, Higaki T, et al. Regulation and characterization of IL-17A expression in patients with chronic rhinosinusitis and its relationship with eosinophilic inflammation. J Allergy Clin Immunol. 2010; Aug. 126(2):397–400.e1-11.
Article44. Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008; Nov. 178(10):1023–32.
Article45. Jeanson L, Kelly M, Coste A, Guerrera IC, Fritsch J, Nguyen-Khoa T, et al. Oxidative stress induces unfolding protein response and inflammation in nasal polyposis. Allergy. 2012; Mar. 67(3):403–12.
Article46. Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiol Rev. 2019; Apr. 99(2):1223–48.
Article47. Hamilos DL. Host-microbial interactions in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2014; Mar. 133(3):640–53.e4.
Article48. Mahdavinia M, Keshavarzian A, Tobin MC, Landay AL, Schleimer RP. A comprehensive review of the nasal microbiome in chronic rhinosinusitis (CRS). Clin Exp Allergy. 2016; Jan. 46(1):21–41.
Article49. Dobretsov K, Negm H, Ralli M, Passali D. The theory of a “staphylococcus superantigen” in chronic rhinosinusitis with nasal polyps: myth or reality. Eur Rev Med Pharmacol Sci. 2019; Mar. 23(1 Suppl):48–54.50. Mahdavinia M, Engen PA, LoSavio PS, Naqib A, Khan RJ, Tobin MC, et al. The nasal microbiome in patients with chronic rhinosinusitis: analyzing the effects of atopy and bacterial functional pathways in 111 patients. J Allergy Clin Immunol. 2018; Jul. 142(1):287–90.
Article51. Sanclement JA, Webster P, Thomas J, Ramadan HH. Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. Laryngoscope. 2005; Apr. 115(4):578–82.
Article52. Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002; Apr. 15(2):167–93.
Article53. Galli J, Calo L, Ardito F, Imperiali M, Bassotti E, Passali GC, et al. Damage to ciliated epithelium in chronic rhinosinusitis: what is the role of bacterial biofilms. Ann Otol Rhinol Laryngol. 2008; Dec. 117(12):902–8.
Article54. Wang X, Du J, Zhao C. Bacterial biofilms are associated with inflammatory cells infiltration and the innate immunity in chronic rhinosinusitis with or without nasal polyps. Inflammation. 2014; Jun. 37(3):871–9.
Article55. Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008; Aug. 112(4):935–45.
Article56. Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005; Apr. 23(1):197–223.
Article57. Nasser A, Moradi M, Jazireian P, Safari H, Alizadeh-Sani M, Pourmand MR, et al. Staphylococcus aureus versus neutrophil: scrutiny of ancient combat. Microb Pathog. 2019; Jun. 131:259–69.
Article58. Chen Y, Lu S, Zhang Y, Yu J, Deng L, Chen H, et al. TLR2 agonist Pam3CSK4 enhances the antibacterial functions of GM-CSF induced neutrophils to methicillin-resistant Staphylococcus aureus. Microb Pathog. 2019; May. 130:204–12.
Article59. Grunwell JR, Stephenson ST, Tirouvanziam R, Brown LA, Brown MR, Fitzpatrick AM. Children with neutrophil-predominant severe asthma have proinflammatory neutrophils with enhanced survival and impaired clearance. J Allergy Clin Immunol. Pract. 2019; Feb. 7(2):516–25.e6.60. Sheshachalam A, Srivastava N, Mitchell T, Lacy P, Eitzen G. Granule protein processing and regulated secretion in neutrophils. Front Immunol. 2014; Sep. 5:448.
Article61. Korkmaz B, Horwitz MS, Jenne DE, Gauthier F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol Rev. 2010; Dec. 62(4):726–59.
Article62. Clancy DM, Sullivan GP, Moran HB, Henry CM, Reeves EP, McElvaney NG, et al. Extracellular neutrophil proteases are efficient regulators of IL-1, IL-33, and IL-36 cytokine activity but poor effectors of microbial killing. Cell Rep. 2018; Mar. 22(11):2937–50.
Article63. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018; Feb. 18(2):134–47.
Article64. Castanheira FV, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019; May. 133(20):2178–85.
Article65. Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014; Nov. 15(11):1017–25.
Article66. Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011; Apr. 472(7344):471–5.
Article67. McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012; Sep. 12(3):324–33.
Article68. Wright TK, Gibson PG, Simpson JL, McDonald VM, Wood LG, Baines KJ. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology. 2016; Apr. 21(3):467–75.
Article69. Cao Y, Chen F, Sun Y, Hong H, Wen Y, Lai Y, et al. LL-37 promotes neutrophil extracellular trap formation in chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2019; Jul. 49(7):990–9.
Article70. Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011; Dec. 128(6):1198–206.e1.
Article71. Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009; Aug. 9(8):581–93.
Article72. Hong CW. Extracellular vesicles of neutrophils. Immune Netw. 2018; Dec. 18(6):e43.
Article73. Dalli J, Montero-Melendez T, Norling LV, Yin X, Hinds C, Haskard D, et al. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol Cell Proteomics. 2013; Aug. 12(8):2205–19.
Article74. Lim K, Hyun YM, Lambert-Emo K, Capece T, Bae S, Miller R, et al. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science. 2015; Sep. 349(6252)::aaa4352.
Article75. Hess C, Sadallah S, Hefti A, Landmann R, Schifferli JA. Ectosomes released by human neutrophils are specialized functional units. J Immunol. 1999; Oct. 163(8):4564–73.
Article76. Prakash PS, Caldwell CC, Lentsch AB, Pritts TA, Robinson BR. Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J Trauma Acute Care Surg. 2012; Aug. 73(2):401–6.
Article77. Genschmer KR, Russell DW, Lal C, Szul T, Bratcher PE, Noerager BD, et al. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell. 2019; Jan. 176:113–26.e15.
Article78. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011; Jul. 11(8):519–31.
Article79. Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005; May. 5(5):375–86.
Article80. Wang BF, Cao PP, Wang ZC, Li ZY, Wang ZZ, Ma J, et al. Interferonγ-induced insufficient autophagy contributes to p62-dependent apoptosis of epithelial cells in chronic rhinosinusitis with nasal polyps. Allergy. 2017; Sep. 72(9):1384–97.
Article81. Lee M, Kim DW, Khalmuratova R, Shin SH, Kim YM, Han DH, et al. The IFN-γ-p38, ERK kinase axis exacerbates neutrophilic chronic rhinosinusitis by inducing the epithelial-to-mesenchymal transition. Mucosal Immunol. 2019; May. 12(3):601–11.
Article82. Sun B, Zhu L, Tao Y, Sun HX, Li Y, Wang P, et al. Characterization and allergic role of IL-33-induced neutrophil polarization. Cell Mol Immunol. 2018; Aug. 15(8):782–93.
Article83. Petersen BC, Budelsky AL, Baptist AP, Schaller MA, Lukacs NW. Interleukin-25 induces type 2 cytokine production in a steroid-resistant interleukin-17RB+ myeloid population that exacerbates asthmatic pathology. Nat Med. 2012; May. 18(5):751–8.
Article84. Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, et al. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res. 2016; May. 110(1):51–61.
Article85. Martin SJ. Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J. 2016; Jul. 283(14):2599–615.
Article86. Kim DK, Eun KM, Kim MK, Cho D, Han SA, Han SY, et al. Comparison between signature cytokines of nasal tissues in subtypes of chronic rhinosinusitis. Allergy Asthma Immunol Res. 2019; Mar. 11(2):201–11.
Article87. Cayrol C, Girard JP. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev. 2018; Jan. 281(1):154–68.
Article88. Hueber AJ, Alves-Filho JC, Asquith DL, Michels C, Millar NL, Reilly JH, et al. IL-33 induces skin inflammation with mast cell and neutrophil activation. Eur J Immunol. 2011; Aug. 41(8):2229–37.
Article89. Alves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA Jr, Auxiliadora-Martins M, et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010; Jun. 16(6):708–12.
Article90. Kim DK, Jin HR, Eun KM, Mo JH, Cho SH, Oh S, et al. The role of interleukin-33 in chronic rhinosinusitis. Thorax. 2017; Jul. 72(7):635–45.
Article91. Liao B, Cao PP, Zeng M, Zhen Z, Wang H, Zhang YN, et al. Interaction of thymic stromal lymphopoietin, IL-33, and their receptors in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. Allergy. 2015; Sep. 70(9):1169–80.
Article92. Baba S, Kondo K, Kanaya K, Suzukawa K, Ushio M, Urata S, et al. Expression of IL-33 and its receptor ST2 in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2014; Apr. 124(4):E115–22.
Article93. Kim DW, Kim DK, Jo A, Jin HR, Eun KM, Mo JH, et al. Age-related decline of neutrophilic inflammation is associated with better postoperative prognosis in non-eosinophilic nasal polyps. PLoS One. 2016; Feb. 11(2):e0148442.
Article94. Morse JC, Li P, Ely KA, Shilts MH, Wannemuehler TJ, Huang LC, et al. Chronic rhinosinusitis in elderly patients is associated with an exaggerated neutrophilic proinflammatory response to pathogenic bacteria. J Allergy Clin Immunol. 2019; Mar. 143(3):990–1002.e6.
Article95. Cho SH, Hong SJ, Han B, Lee SH, Suh L, Norton J, et al. Age-related differences in the pathogenesis of chronic rhinosinusitis. J Allergy Clin Immunol. 2012; Mar. 129(3):858–60.e2.
Article96. Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils: separation of survival and activation outcomes. J Immunol. 1995; May. 154(9):4719–25.97. Tamaoki J. The effects of macrolides on inflammatory cells. Chest. 2004; Feb. 125(2 Suppl):41S–50S.
Article98. Tauber SC, Nau R. Immunomodulatory properties of antibiotics. Curr Mol Pharmacol. 2008; Jan. 1(1):68–79.99. Luo Q, Chen F, Liu W, Li Z, Xu R, Fan Y, et al. Evaluation of longterm clarithromycin treatment in adult Chinese patients with chronic rhinosinusitis without nasal polyps. ORL J Otorhinolaryngol Relat Spec. 2011; 73(4):206–11.
Article100. Wallwork B, Coman W, Mackay-Sim A, Greiff L, Cervin A. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope. 2006; Feb. 116(2):189–93.
Article101. Videler WJ, Badia L, Harvey RJ, Gane S, Georgalas C, van der Meulen FW, et al. Lack of efficacy of long-term, low-dose azithromycin in chronic rhinosinusitis: a randomized controlled trial. Allergy. 2011; Nov. 66(11):1457–68.
Article102. Rennard SI, Dale DC, Donohue JF, Kanniess F, Magnussen H, Sutherland ER, et al. CXCR2 antagonist MK-7123: a phase 2 proof-of-concept trial for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015; May. 191(9):1001–11.
Article103. Todd CM, Salter BM, Murphy DM, Watson RM, Howie KJ, Milot J, et al. The effects of a CXCR1/CXCR2 antagonist on neutrophil migration in mild atopic asthmatic subjects. Pulm Pharmacol Ther. 2016; Dec. 41:34–9.
Article104. Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, et al. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013; Feb. 20(2):188–201.
Article105. Natsis NE, Gottlieb AB. Bimekizumab for the treatment of psoriatic disease. Expert Opin Biol Ther. 2018; Dec. 18(12):1193–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medical treatment according to phenotypes of chronic rhinosinusitis
- Can Neutrophils Be a Cellular Biomarker in Asian Chronic Rhinosinusitis?
- Pathogenesis of Recalcitrant Chronic Rhinosinusitis: The Emerging Role of Innate Immune Cells
- Roles of IL-8 in the Pathogenesis of Chronic Rhinosinusitis
- Clinical Characteristics and Treatment of Fungal Rhinosinusitis