J Korean Assoc Oral Maxillofac Surg.
2019 Oct;45(5):285-293. 10.5125/jkaoms.2019.45.5.285.
Reduction in post extraction waiting period for dental implant patients using plasma rich in growth factors: an in vivo study using cone-beam computed tomography
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Faculty of Dental Sciences, SGT University, Gurgaon, India.
- 2Department of Dentistry, Shaheed Hasan Khan Mewati (SHKM), Govt. Medical College, Nalhar, Nuh, Mewat, India. vijay_laxmy13@yahoo.co.in
- 3Private Clinic, New Delhi, India.
- KMID: 2461481
- DOI: http://doi.org/10.5125/jkaoms.2019.45.5.285
Abstract
OBJECTIVES
This study examined the effects of plasma-rich growth factors (PRGF) on accelerating bone regeneration/repair in fresh extraction sockets, and determined the quality and quantity of bone by assessing the bone density using cone-beam computed tomography (CBCT).
MATERIALS AND METHODS
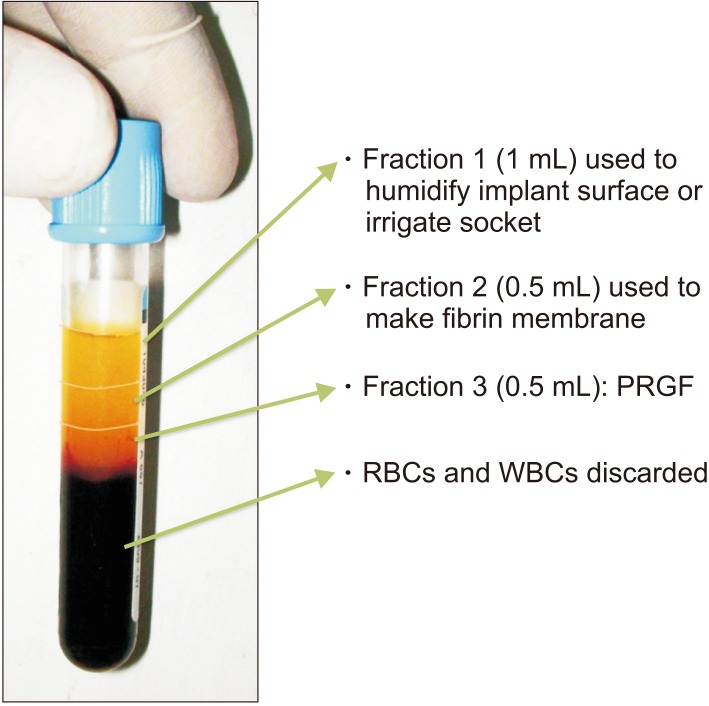
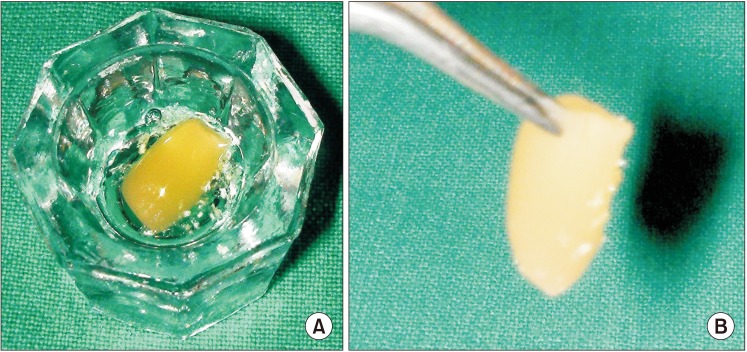
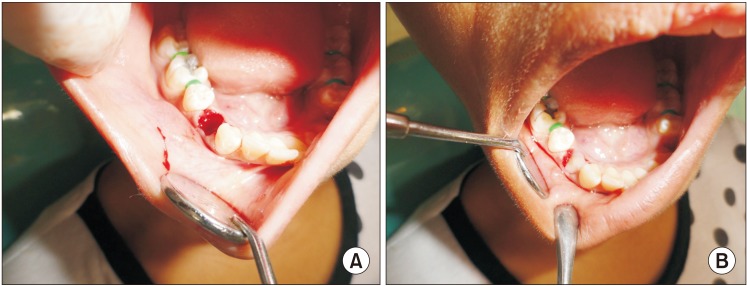
Twenty patients, who had undergone bilateral extractions, were included in this study. In one extraction socket, PRGF was used and covered with an autologous fibrin plug. Nothing was used in the opposite side extraction socket. Thirteen weeks post extraction, the level of bone regeneration was evaluated on both sides with CBCT.
RESULTS
At the end of the study, the mean bone density according to the Hounsfield units (HU) in the control group and PRGF group was 500.05 HU (type III bone type) and 647.95 HU (type II bone type), respectively.
CONCLUSION
This study recommends the use of PRGF in post extraction sites to accelerate the rate of bone regeneration and improve the quality of regenerated bone. The technique to process PRGF was simple compared to previously mentioned techniques used for platelet-rich plasma (PRP) preparation. PRP preparation requires a two-cycle centrifugation procedure, leading to a longer processing time.
Keyword
MeSH Terms
Figure
Reference
-
1. Thor A, Wannfors K, Sennerby L, Rasmusson L. Reconstruction of the severely resorbed maxilla with autogenous bone, platelet-rich plasma, and implants: 1-year results of a controlled prospective 5-year study. Clin Implant Dent Relat Res. 2005; 7:209–220. PMID: 16336912.2. Del Fabbro M, Boggian C, Taschieri S. Immediate implant placement into fresh extraction sites with chronic periapical pathologic features combined with plasma rich in growth factors: preliminary results of single-cohort study. J Oral Maxillofac Surg. 2009; 67:2476–2484. PMID: 19837321.
Article3. Lekholm U, Zarb GA. Patient selection and preparation. In : Brånemark PI, Zarb GA, Albrektsson T, editors. Tissue-integrated prostheses: osseointegration in clinical dentistry. Chicago: Quintessence;1985. p. 199–209.4. Anitua E, Orive G, Andía I. Use of PRGF to accelerate bone and soft tissue regeneration in postextraction sites: evaluation of regenerated bone density. Implant Dialog. 2003; 36:3–14.5. Irinakis T. Rationale for socket preservation after extraction of a single-rooted tooth when planning for future implant placement. J Can Dent Assoc. 2006; 72:917–922. PMID: 17187706.6. Lekovic V, Camargo PM, Klokkevold PR, Weinlaender M, Kenney EB, Dimitrijevic B, et al. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J Periodontol. 1998; 69:1044–1049. PMID: 9776033.
Article7. Anitua E. The use of plasma-rich growth factors (PRGF) in oral surgery. Pract Proced Aesthet Dent. 2001; 13:487–493. quiz 487-93. PMID: 11544821.8. Anitua E, Alkhraisat MH, Piñas L, Orive G. Efficacy of biologically guided implant site preparation to obtain adequate primary implant stability. Ann Anat. 2015; 199:9–15. PMID: 24661634.
Article9. Deuel TF, Huang JS, Proffitt RT, Baenziger JU, Chang D, Kennedy BB. Human platelet-derived growth factor. Purification and resolution into two active protein fractions. J Biol Chem. 1981; 256:8896–8899. PMID: 7263691.
Article10. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004; 62:489–496. PMID: 15085519.
Article11. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85:638–646. PMID: 9638695.12. Sammartino G, Tia M, Marenzi G, di Lauro AE, D'Agostino E, Claudio PP. Use of autologous platelet-rich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J Oral Maxillofac Surg. 2005; 63:766–770. PMID: 15944972.
Article13. Célio-Mariano R, de Melo WM, Carneiro-Avelino C. Comparative radiographic evaluation of alveolar bone healing associated with autologous platelet-rich plasma after impacted mandibular third molar surgery. J Oral Maxillofac Surg. 2012; 70:19–24. PMID: 21778014.
Article14. Alissa R, Esposito M, Horner K, Oliver R. The influence of platelet-rich plasma on the healing of extraction sockets: an explorative randomised clinical trial. Eur J Oral Implantol. 2010; 3:121–134. PMID: 20623037.15. Antonello Gde M, Torres do Couto R, Giongo CC, Corrêa MB, Chagas Júnior OL, Lemes CH. Evaluation of the effects of the use of platelet-rich plasma (PRP) on alveolar bone repair following extraction of impacted third molars: prospective study. J Craniomaxillofac Surg. 2013; 41:e70–e75. PMID: 23352081.16. Kawase T. Platelet-rich plasma and its derivatives as promising bioactive materials for regenerative medicine: basic principles and concepts underlying recent advances. Odontology. 2015; 103:126–135. PMID: 26040505.
Article17. Muntean W, Zenz W, Finding K, Zobel G, Beitzke A. Inhibitor to factor V after exposure to fibrin sealant during cardiac surgery in a two-year-old child. Acta Paediatr. 1994; 83:84–87. PMID: 8193480.
Article18. Weibrich G, Kleis WK, Hitzler WE, Hafner G. Comparison of the platelet concentrate collection system with the plasma-richin-growth-factors kit to produce platelet-rich plasma: a technical report. Int J Oral Maxillofac Implants. 2005; 20:118–123. PMID: 15747683.19. Gürbüzer B, Pikdöken L, Tunali M, Urhan M, Küçükodaci Z, Ercan F. Scintigraphic evaluation of osteoblastic activity in extraction sockets treated with platelet-rich fibrin. J Oral Maxillofac Surg. 2010; 68:980–989. PMID: 20144497.
Article20. Nishiyama K, Okudera T, Watanabe T, Isobe K, Suzuki M, Masuki H, et al. Basic characteristics of plasma rich in growth factors (PRGF): blood cell components and biological effects. Clin Exp Dent Res. 2016; 2:96–103. PMID: 29744155.
Article21. Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:e56–e60. PMID: 16504852.
Article22. Al-Hamed FS, Tawfik MA, Abdelfadil E, Al-Saleh MAQ. Efficacy of platelet-rich fibrin after mandibular third molar extraction: a systematic review and meta-analysis. J Oral Maxillofac Surg. 2017; 75:1124–1135. PMID: 28236425.
Article23. Choukroun J. Advanced PRF, & i-PRF: platelet concentrates or blood concentrates? J Periodontal Med Clin Pract. 2014; 1:3.24. Masuki H, Okudera T, Watanebe T, Suzuki M, Nishiyama K, Okudera H, et al. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int J Implant Dent. 2016; 2:19. PMID: 27747711.
Article25. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009; 27:158–167. PMID: 19187989.
Article26. Magalon J, Chateau AL, Bertrand B, Louis ML, Silvestre A, Giraudo L, et al. DEPA classification: a proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc Med. 2016; 2:e000060.
Article27. Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999; 14:529–535. PMID: 10453668.28. Fuerst G, Gruber R, Tangl S, Sanroman F, Watzek G. Enhanced bone-to-implant contact by platelet-released growth factors in mandibular cortical bone: a histomorphometric study in minipigs. Int J Oral Maxillofac Implants. 2003; 18:685–690. PMID: 14579956.29. Anitua E, Murias-Freijo A, Alkhraisat MH, Orive G. Clinical, radiographical, and histological outcomes of plasma rich in growth factors in extraction socket: a randomized controlled clinical trial. Clin Oral Investig. 2015; 19:589–600.
Article30. Wang HL, Pappert TD, Castelli WA, Chiego DJ Jr, Shyr Y, Smith BA. The effect of platelet-derived growth factor on the cellular response of the periodontium: an autoradiographic study on dogs. J Periodontol. 1994; 65:429–436. PMID: 8046558.
Article31. Gruber R, Varga F, Fischer MB, Watzek G. Platelets stimulate proliferation of bone cells: involvement of platelet-derived growth factor, microparticles and membranes. Clin Oral Implants Res. 2002; 13:529–535. PMID: 12453131.
Article32. Weibrich G, Gnoth SH, Otto M, Reichert TE, Wagner W. [Growth stimulation of human osteoblast-like cells by thrombocyte concentrates in vitro]. Mund Kiefer Gesichtschir. 2002; 6:168–174. German. PMID: 12143129.33. Anitua E, Prado R, Troya M, Zalduendo M, de la Fuente M, Pino A, et al. Implementation of a more physiological plasma rich in growth factor (PRGF) protocol: Anticoagulant removal and reduction in activator concentration. Platelets. 2016; 27:459–466. PMID: 26940906.
Article34. Mozzati M, Gallesio G, di Romana S, Bergamasco L, Pol R. Efficacy of plasma-rich growth factor in the healing of postextraction sockets in patients affected by insulin-dependent diabetes mellitus. J Oral Maxillofac Surg. 2014; 72:456–462. PMID: 24342581.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of root canal perforation by using cone-beam computed tomography
- Horizontal alteration of anterior alveolar ridge after immediate implant placement: A retrospective cone beam computed tomography analysis
- Commentary on "Reliability of two different presurgical preparation methods for implant dentistry based on panoramic radiography and cone-beam computed tomography in cadavers"
- Reply on "Reliability of two different presurgical preparation methods for implant dentistry based on panoramic radiography and cone-beam computed tomography in cadavers"
- Three-dimensional imaging modalities in endodontics