Brain Tumor Res Treat.
2019 Oct;7(2):98-104. 10.14791/btrt.2019.7.e38.
Experience Profiling of Fluorescence-Guided Surgery I: Gliomas
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea. nschpark@snu.ac.kr
- 2Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2461182
- DOI: http://doi.org/10.14791/btrt.2019.7.e38
Abstract
- BACKGROUND
Numerous studies reported a usefulness of 5-aminolevulinic acid (5-ALA) fluorescence-guided surgery (FGS) in high grade gliomas. However, fluorescence patterns and intensities are variable among gliomas. In this study, we report our extensive experience with FGS in various gliomas, focusing on epidemiological data of fluorescence patterns.
METHODS
A total of 827 histologically proven glioma patients out of 900 brain tumor patients who had undergone FGS using 5-ALA during the period of 8.5 years between July 2010 and January 2019 were analyzed. Indications of FGS in glioma surgery are evidence for possible high-grade foci in putative gliomas in preoperative MRI.
RESULTS
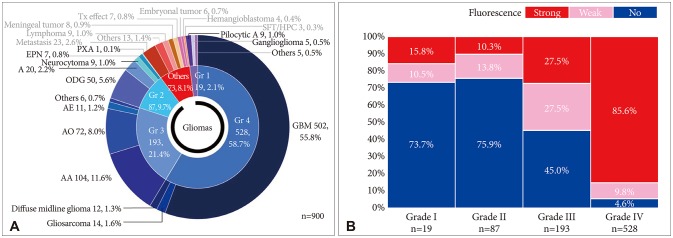
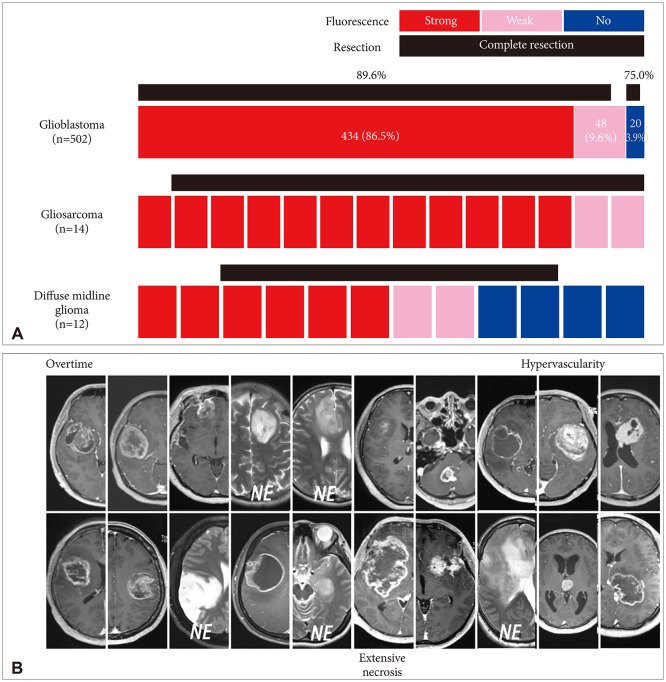
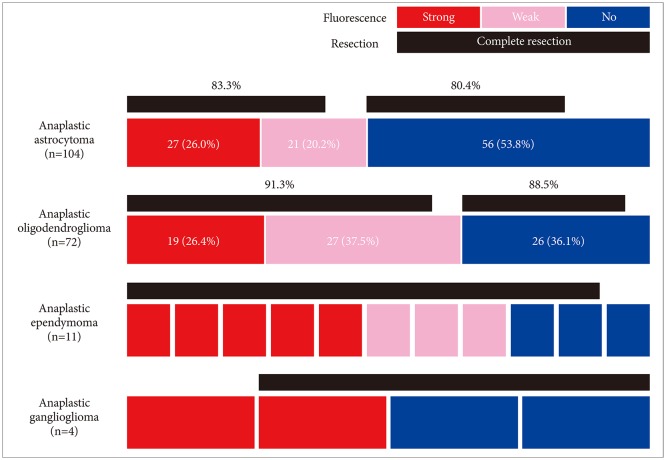
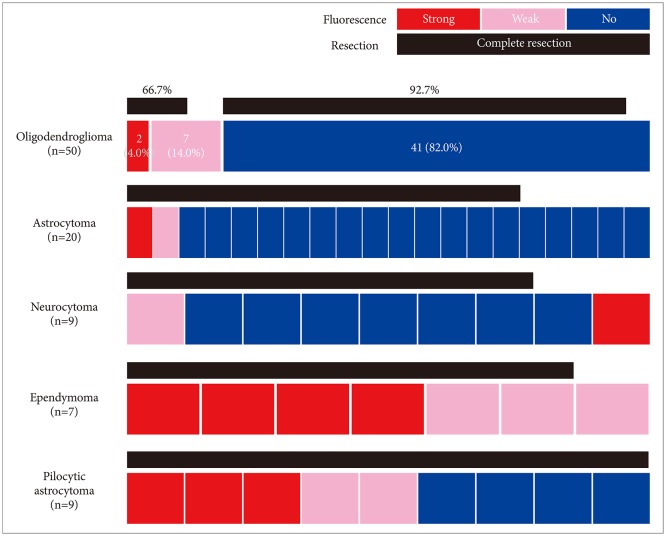
Among the 827 gliomas, the number of cases corresponding to 2016 World Health Organization (WHO) grade IV, III, II, and I are 528 (58.7%), 193 (21.4%), 87 (9.7%) and 19 (2.1%), respectively. In terms of fluorescence rate, grade IV gliomas showed positive fluorescence in 95.4% of cases including strong intensity in 85.6%. Grade III gliomas showed fluorescence in about half of cases (55.0%), but 45.0% of the cases showed no fluorescence at all. Anaplastic oligodendroglioma had a higher positive rate (63.9%) than anaplastic astrocytoma (46.2%). Both grade II and I gliomas still showed positive fluorescence in about one-fourth of cases (24.1% and 26.3% respectively). Among them ependymoma and pilocytic astrocytoma were fluorescence-prone tumors.
CONCLUSION
This epidemiological data of 5-ALA fluorescence in various grades of glioma provides a basic reference to the clinical application of FGS with 5-ALA in glioma surgery.
Keyword
MeSH Terms
Figure
Reference
-
1. McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008; 63:700–707. author reply 707-8. PMID: 18981880.
Article2. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008; 26:1338–1345. PMID: 18323558.
Article3. Stummer W, Kamp MA. The importance of surgical resection in malignant glioma. Curr Opin Neurol. 2009; 22:645–649. PMID: 19738467.
Article4. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10:459–466. PMID: 19269895.5. Brown PD, Maurer MJ, Rummans TA, et al. A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: the impact of the extent of resection on quality of life and survival. Neurosurgery. 2005; 57:495–504. PMID: 16145528.
Article6. Stepp H, Stummer W. 5-ALA in the management of malignant glioma. Lasers Surg Med. 2018; 50:399–419. PMID: 29737540.
Article7. Teng L, Nakada M, Zhao SG, et al. Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br J Cancer. 2011; 104:798–807. PMID: 21304523.
Article8. Wolburg H, Noell S, Fallier-Becker P, Mack AF, Wolburg-Buchholz K. The disturbed blood-brain barrier in human glioblastoma. Mol Aspects Med. 2012; 33:579–589. PMID: 22387049.
Article9. Zhao SG, Chen XF, Wang LG, et al. Increased expression of ABCB6 enhances protoporphyrin IX accumulation and photodynamic effect in human glioma. Ann Surg Oncol. 2013; 20:4379–4388. PMID: 22688660.
Article10. Ferraro N, Barbarite E, Albert TR, et al. The role of 5-aminolevulinic acid in brain tumor surgery: a systematic review. Neurosurg Rev. 2016; 39:545–555. PMID: 26815631.
Article11. Stummer W, Stocker S, Novotny A, et al. In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J Photochem Photobiol B. 1998; 45:160–169. PMID: 9868806.
Article12. Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016; 130:269–282. PMID: 27174197.
Article13. Kim SK, Choi SH, Kim YH, Park CK. Impact of fluorescence-guided surgery on the improvement of clinical outcomes in glioblastoma patients. Neurooncol Pract. 2014; 1:81–85. PMID: 31386036.
Article14. Roberts DW, Valdés PA, Harris BT, et al. Glioblastoma multiforme treatment with clinical trials for surgical resection (aminolevulinic acid). Neurosurg Clin N Am. 2012; 23:371–377. PMID: 22748650.
Article15. Stummer W, Tonn JC, Mehdorn HM, et al. Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Clinical article. J Neurosurg. 2011; 114:613–623. PMID: 20397896.16. Zhang C, Boop FA, Ruge J. The use of 5-aminolevulinic acid in resection of pediatric brain tumors: a critical review. J Neurooncol. 2019; 141:567–573. PMID: 30443833.
Article17. Jaber M, Wölfer J, Ewelt C, et al. The value of 5-aminolevulinic acid in low-grade gliomas and high-grade gliomas lacking glioblastoma imaging features: an analysis based on fluorescence, magnetic resonance imaging, 18F-fluoroethyl tyrosine positron emission tomography, and tumor molecular factors. Neurosurgery. 2016; 78:401–411. PMID: 26366972.
Article18. Kim S, Kim JE, Kim YH, et al. Glutaminase 2 expression is associated with regional heterogeneity of 5-aminolevulinic acid fluorescence in glioblastoma. Sci Rep. 2017; 7:12221. PMID: 28939850.
Article19. Roessler K, Becherer A, Donat M, Cejna M, Zachenhofer I. Intraoperative tissue fluorescence using 5-aminolevolinic acid (5-ALA) is more sensitive than contrast MRI or amino acid positron emission tomography ((18)F-FET PET) in glioblastoma surgery. Neurol Res. 2012; 34:314–317. PMID: 22449387.
Article20. Schucht P, Knittel S, Slotboom J, et al. 5-ALA complete resections go beyond MR contrast enhancement: shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir (Wien). 2014; 156:305–312. discussion 312. PMID: 24449075.
Article21. Hadjipanayis CG, Widhalm G, Stummer W. What is the surgical benefit of utilizing 5-aminolevulinic acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery. 2015; 77:663–673. PMID: 26308630.
Article22. Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. 2014; 32:774–782. PMID: 24516010.
Article23. McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009; 110:156–162. PMID: 18847342.
Article24. Díez Valle R, Hadjipanayis CG, Stummer W. Established and emerging uses of 5-ALA in the brain: an overview. J Neurooncol. 2019; 141:487–494. PMID: 30607705.
Article25. Kim JE, Cho HR, Xu WJ, et al. Mechanism for enhanced 5-aminolevulinic acid fluorescence in isocitrate dehydrogenase 1 mutant malignant gliomas. Oncotarget. 2015; 6:20266–20277. PMID: 26008980.
Article26. Saito K, Hirai T, Takeshima H, et al. Genetic factors affecting intraoperative 5-aminolevulinic acid-induced fluorescence of diffuse gliomas. Radiol Oncol. 2017; 51:142–150. PMID: 28740449.
Article27. Haj-Hosseini N, Richter JC, Hallbeck M, Wårdell K. Low dose 5-aminolevulinic acid: implications in spectroscopic measurements during brain tumor surgery. Photodiagnosis Photodyn Ther. 2015; 12:209–214. PMID: 25818546.
Article28. Chung IW, Eljamel S. Risk factors for developing oral 5-aminolevulinic acid-induced side effects in patients undergoing fluorescence guided resection. Photodiagnosis Photodyn Ther. 2013; 10:362–367. PMID: 24284086.29. GmbH M. Gliolan(R) product information. Wedel: Medac;2014. Accessed September 23, 2015. at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000744/WC500021790.pdf.30. Valdés PA, Leblond F, Kim A, et al. Quantitative fluorescence in intracranial tumor: implications for ALA-induced PpIX as an intraoperative biomarker. J Neurosurg. 2011; 115:11–17. PMID: 21438658.
Article31. Valdés PA, Kim A, Leblond F, et al. Combined fluorescence and reflectance spectroscopy for in vivo quantification of cancer biomarkers in low- and high-grade glioma surgery. J Biomed Opt. 2011; 16:116007. PMID: 22112112.32. Nishikawa R. Fluorescence illuminates the way…. Neuro Oncol. 2011; 13:805. PMID: 21798845.33. Widhalm G, Kiesel B, Woehrer A, et al. 5-Aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS One. 2013; 8:e76988. PMID: 24204718.
Article34. Marbacher S, Klinger E, Schwyzer L, et al. Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus. 2014; 36:E10.
Article35. Schwake M, Schipmann S, Müther M, Köchling M, Brentrup A, Stummer W. 5-ALA fluorescence-guided surgery in pediatric brain tumors-a systematic review. Acta Neurochir (Wien). 2019; 161:1099–1108. PMID: 30989383.
Article36. Ewelt C, Floeth FW, Felsberg J, et al. Finding the anaplastic focus in diffuse gliomas: the value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin Neurol Neurosurg. 2011; 113:541–547. PMID: 21507562.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experience Profiling of Fluorescence-Guided Surgery II: Non-Glioma Pathologies
- Fluorescence Guided Surgery with 5-Aminolevulinic Acid for Resection of Spinal Cord Ependymomas
- 5-Aminolevulinic Acid Fluorescence Discriminates the Histological Grade of Extraventricular Neurocytoma
- Diagnostic potential of laser-induced autofluorescence emission in brain tissue
- Indocyanine Green-Guided Video-Assisted Thoracoscopic Surgery for Resection of an Ectopic Mediastinal Parathyroid Adenoma