Brain Tumor Res Treat.
2019 Oct;7(2):105-111. 10.14791/btrt.2019.7.e39.
Experience Profiling of Fluorescence-Guided Surgery II: Non-Glioma Pathologies
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea. nschpark@snu.ac.kr
- 2Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2461183
- DOI: http://doi.org/10.14791/btrt.2019.7.e39
Abstract
- BACKGROUND
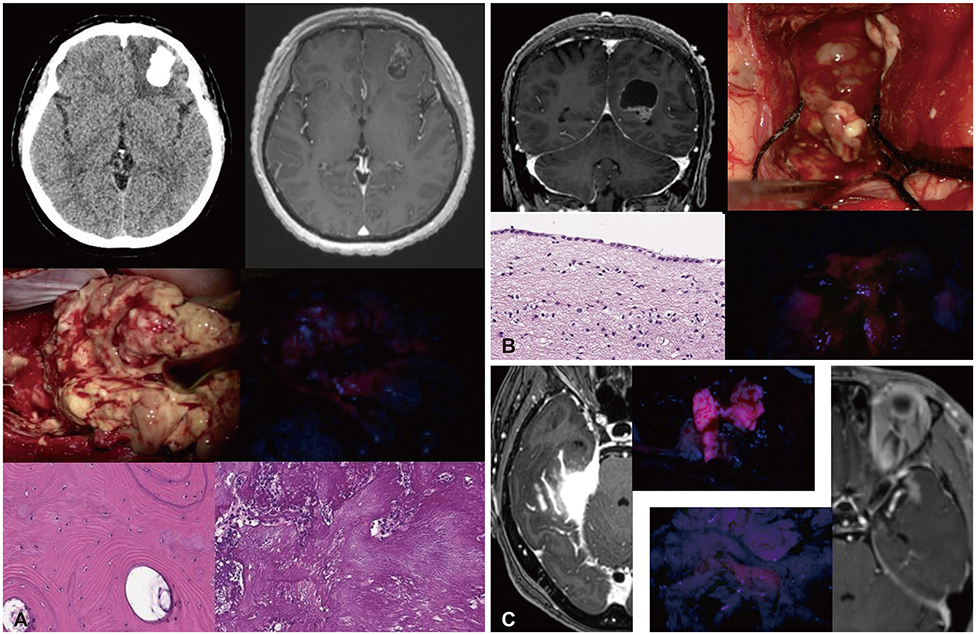
Only sporadic reports of fluorescence-guided surgery (FGS) have been published for non-glioma conditions. In this study, we focus on epidemiological data of fluorescence patterns and report the diverse experiences of FGS in non-gliomas.
METHODS
During 8.5 years between July 2010 and January 2019, 900 FGS for brain tumor performed in Seoul National University Hospital. Among them, a total of 73 histologically proven non-glioma patients were analyzed. Indications for FGS have been the possibility of anaplastic tumor in intra-axial tumors in preoperative MRI and an attempt to reproduce known anecdotal experiences of 5-Aminolevulinic Acid (5-ALA) fluorescence.
RESULTS
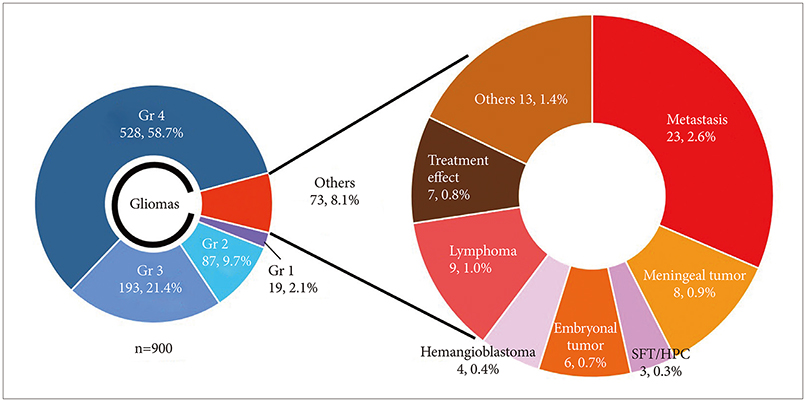
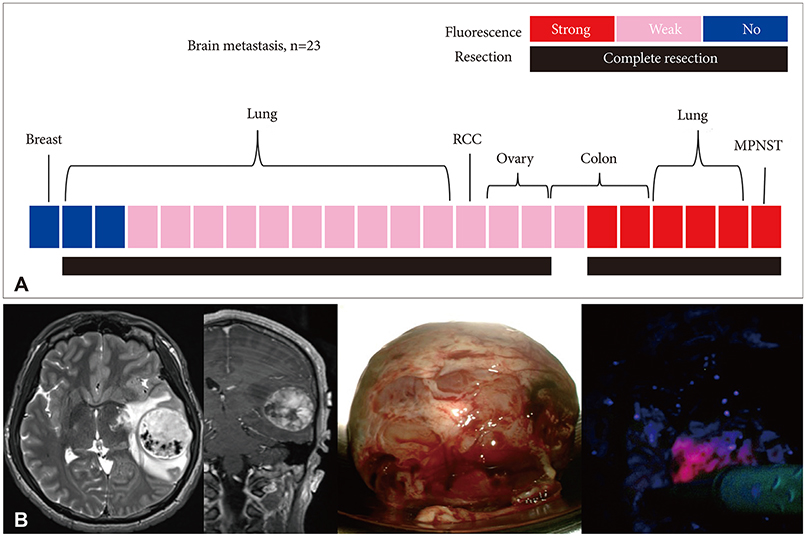
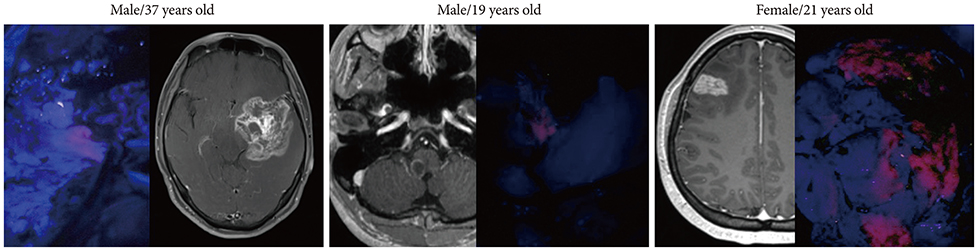
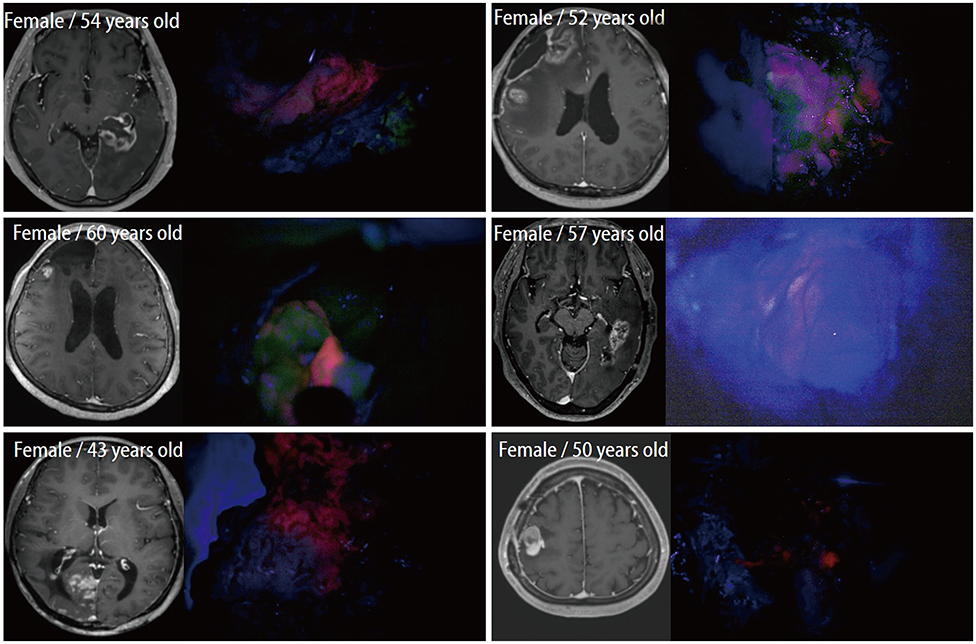
In cases of brain tumors except for gliomas, the most frequent cases were brain metastasis (23 cases) followed by lymphomas (9 cases) and meningeal tumors (8 cases). And there were embryonal tumors (6 cases), hemangioblastomas (4 cases), and solitary fibrous tumor/hemangiopericytomas (3 cases). Most brain metastases, meningiomas, primary central nervous system lymphomas, and treatment effect cases showed positive fluorescence. Moreover, some non-tumorous conditions also showed positive fluorescence. However, hemangioblastoma and germ cell tumor did not observe any fluorescence at all.
CONCLUSION
5-ALA induced fluorescence is not limited to glioma but is also evident in non-glioma and non-neoplastic conditions. This 5-ALA-induced fluorescence may be used as an intraoperative tool for various brain conditions.
Keyword
MeSH Terms
Figure
Reference
-
1. Hadjipanayis CG, Widhalm G, Stummer W. What is the surgical benefit of utilizing 5-aminolevulinic acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery. 2015; 77:663–673.
Article2. Ewelt C, Nemes A, Senner V, et al. Fluorescence in neurosurgery: Its diagnostic and therapeutic use. Review of the literature. J Photochem Photobiol B. 2015; 148:302–309.
Article3. Chae MP, Song SW, Park SH, Park CK. Experience with 5-aminolevulinic acid in fluorescence-guided resection of a deep sylvian meningioma. J Korean Neurosurg Soc. 2012; 52:558–560.
Article4. Valdes PA, Bekelis K, Harris BT, et al. 5-Aminolevulinic acid-induced protoporphyrin IX fluorescence in meningioma: qualitative and quantitative measurements in vivo. Neurosurgery. 2014; 10 Suppl 1:74–82.
Article5. Grimbergen MC, van Swol CF, van Moorselaar RJ, Uff J, Mahadevan-Jansen A, Stone N. Raman spectroscopy of bladder tissue in the presence of 5-aminolevulinic acid. J Photochem Photobiol B. 2009; 95:170–176.
Article6. Huber RM, Gamarra F, Hautmann H, et al. 5-aminolaevulinic acid (ALA) for the fluorescence detection of bronchial tumors. Diagn Ther Endosc. 1999; 5:113–118.
Article7. Baas P, Triesscheijn M, Burgers S, van Pel R, Stewart F, Aalders M. Fluorescence detection of pleural malignancies using 5-aminolaevulinic acid. Chest. 2006; 129:718–724.
Article8. Millesi M, Kiesel B, Woehrer A, et al. Analysis of 5-aminolevulinic acid-induced fluorescence in 55 different spinal tumors. Neurosurg Focus. 2014; 36:E11.
Article9. Kamp MA, Munoz-Bendix C, Mijderwijk HJ, et al. Is 5-ALA fluorescence of cerebral metastases a prognostic factor for local recurrence and overall survival. J Neurooncol. 2019; 141:547–553.
Article10. Utsuki S, Miyoshi N, Oka H, et al. Fluorescence-guided resection of metastatic brain tumors using a 5-aminolevulinic acid-induced protoporphyrin IX: pathological study. Brain Tumor Pathol. 2007; 24:53–55.
Article11. Kamp MA, Grosser P, Felsberg J, et al. 5-aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study. Acta Neurochir (Wien). 2012; 154:223–228.
Article12. Marbacher S, Klinger E, Schwyzer L, et al. Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus. 2014; 36:E10.
Article13. Kamp MA, Fischer I, Bühner J, et al. 5-ALA fluorescence of cerebral metastases and its impact for the local-in-brain progression. Oncotarget. 2016; 7:66776–66789.
Article14. Teng L, Nakada M, Zhao SG, et al. Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br J Cancer. 2011; 104:798–807.
Article15. Utsuki S, Oka H, Sato S, et al. Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol Med Chir (Tokyo). 2007; 47:210–213.
Article16. Valdes PA, Millesi M, Widhalm G, Roberts DW. 5-aminolevulinic acid induced protoporphyrin IX (ALA-PpIX) fluorescence guidance in meningioma surgery. J Neurooncol. 2019; 141:555–565.
Article17. Potapov AA, Goryaynov SA, Okhlopkov VA, et al. Laser biospectroscopy and 5-ALA fluorescence navigation as a helpful tool in the meningioma resection. Neurosurg Rev. 2016; 39:437–447.
Article18. Morofuji Y, Matsuo T, Hayashi Y, Suyama K, Nagata I. Usefulness of intraoperative photodynamic diagnosis using 5-aminolevulinic acid for meningiomas with cranial invasion: technical case report. Neurosurgery. 2008; 62:3 Suppl 1. 102–103.19. Kajimoto Y, Kuroiwa T, Miyatake S, et al. Use of 5-aminolevulinic acid in fluorescence-guided resection of meningioma with high risk of recurrence. Case report. J Neurosurg. 2007; 106:1070–1074.
Article20. Coluccia D, Fandino J, Fujioka M, Cordovi S, Muroi C, Landolt H. Intraoperative 5-aminolevulinic-acid-induced fluorescence in meningiomas. Acta Neurochir (Wien). 2010; 152:1711–1719.
Article21. Cornelius JF, Slotty PJ, Kamp MA, Schneiderhan TM, Steiger HJ, El-Khatib M. Impact of 5-aminolevulinic acid fluorescence-guided surgery on the extent of resection of meningiomas--with special regard to high-grade tumors. Photodiagnosis Photodyn Ther. 2014; 11:481–490.
Article22. Foster N, Eljamel S. ALA-induced fluorescence image guided surgery of meningiomas: a meta-analyses. Photodiagnosis Photodyn Ther. 2016; 15:73–78.
Article23. Millesi M, Kiesel B, Mischkulnig M, et al. Analysis of the surgical benefits of 5-ALA-induced fluorescence in intracranial meningiomas: experience in 204 meningiomas. J Neurosurg. 2016; 125:1408–1419.
Article24. Della Puppa A, Rustemi O, Gioffrè G, et al. Predictive value of intraoperative 5-aminolevulinic acid-induced fluorescence for detecting bone invasion in meningioma surgery. J Neurosurg. 2014; 120:840–845.
Article25. Valdés PA, Leblond F, Kim A, et al. Quantitative fluorescence in intracranial tumor: implications for ALA-induced PpIX as an intraoperative biomarker. J Neurosurg. 2011; 115:11–17.
Article26. Yamamoto J, Kitagawa T, Akiba D, Nishizawa S. 5-Aminolevulinic acid-induced fluorescence in cerebellar primary central nervous system lymphoma: a case report and literature review. Turk Neurosurg. 2015; 25:796–800.
Article27. Kiesel B, Millesi M, Woehrer A, et al. 5-ALA-induced fluorescence as a marker for diagnostic tissue in stereotactic biopsies of intracranial lymphomas: experience in 41 patients. Neurosurg Focus. 2018; 44:E7.
Article28. Takada T, Tamura M, Yamamoto T, Matsui H, Matsumura A. Selective accumulation of hematoporphyrin derivative in glioma through proton-coupled folate transporter SLC46A1. J Clin Biochem Nutr. 2014; 54:26–30.
Article29. Desmoulin SK, Hou Z, Gangjee A, Matherly LH. The human protoncoupled folate transporter: Biology and therapeutic applications to cancer. Cancer Biol Ther. 2012; 13:1355–1373.30. Evers G, Kamp M, Warneke N, et al. 5-Aminolaevulinic Acid-Induced Fluorescence in Primary Central Nervous System Lymphoma. World Neurosurg. 2017; 98:375–380.
Article31. El-Sharabasy MM, el-Waseef AM, Hafez MM, Salim SA. Porphyrin metabolism in some malignant diseases. Br J Cancer. 1992; 65:409–412.
Article32. Rittenhouse-Diakun K, Van Leengoed H, Morgan J, et al. The role of transferrin receptor (CD71) in photodynamic therapy of activated and malignant lymphocytes using the heme precursor delta-aminolevulinic acid (ALA). Photochem Photobiol. 1995; 61:523–528.
Article33. Yamamoto T, Ishikawa E, Miki S, et al. Photodynamic diagnosis using 5-aminolevulinic acid in 41 biopsies for primary central nervous system lymphoma. Photochem Photobiol. 2015; 91:1452–1457.
Article34. Utsuki S, Oka H, Kijima C, Miyajima Y, Hagiwara H, Fujii K. Utility of intraoperative fluorescent diagnosis of residual hemangioblastoma using 5-aminolevulinic acid. Neurol India. 2011; 59:612–615.
Article35. Utsuki S, Oka H, Sato K, Shimizu S, Suzuki S, Fujii K. Fluorescence diagnosis of tumor cells in hemangioblastoma cysts with 5-aminolevulinic acid. J Neurosurg. 2010; 112:130–132.
Article36. Takeda J, Nonaka M, Li Y, et al. 5-ALA fluorescence-guided endoscopic surgery for mixed germ cell tumors. J Neurooncol. 2017; 134:119–124.
Article37. Kamp MA, Felsberg J, Sadat H, et al. 5-ALA-induced fluorescence behavior of reactive tissue changes following glioblastoma treatment with radiation and chemotherapy. Acta Neurochir (Wien). 2015; 157:207–213.
Article38. Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003; 9:180–188.
Article39. Chaskis C, Neyns B, Michotte A, De Ridder M, Everaert H. Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations. Surg Neurol. 2009; 72:423–428.
Article40. Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol. 2008; 10:361–367.
Article41. De Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004; 63:535–537.
Article42. Wong CS, Van der Kogel AJ. Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv. 2004; 4:273–284.
Article43. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008; 9:453–461.
Article44. Nabavi A, Thurm H, Zountsas B, et al. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase ii study. Neurosurgery. 2009; 65:1070–1076.
Article45. Behling F, Hennersdorf F, Bornemann A, Tatagiba M, Skardelly M. 5-Aminolevulinic acid accumulation in a cerebral infarction mimicking high-grade glioma. World Neurosurg. 2016; 92:586.e5–586.e8.
Article46. Nestler U, Warter A, Cabre P, Manzo N. A case of late-onset multiple sclerosis mimicking glioblastoma and displaying intraoperative 5-aminolevulinic acid fluorescence. Acta Neurochir (Wien). 2012; 154:899–901.
Article47. Höhne J, Brawanski A, Schebesch KM. Fluorescein sodium-guided surgery of a brain abscess: a case report. Surg Neurol Int. 2016; 7(Suppl 39):S955–S957.
Article48. Omoto K, Matsuda R, Nakagawa I, Motoyama Y, Nakase H. False-positive inflammatory change mimicking glioblastoma multiforme under 5-aminolevulinic acid-guided surgery: a case report. Surg Neurol Int. 2018; 9:49.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experience Profiling of Fluorescence-Guided Surgery I: Gliomas
- Fluorescence Guided Surgery with 5-Aminolevulinic Acid for Resection of Spinal Cord Ependymomas
- 5-Aminolevulinic Acid Fluorescence Discriminates the Histological Grade of Extraventricular Neurocytoma
- Indocyanine Green-Guided Video-Assisted Thoracoscopic Surgery for Resection of an Ectopic Mediastinal Parathyroid Adenoma
- Clinical Experience of Glioma Surgery Using "Tailed Bullet": Overcoming the Limitations of Conventional Neuro-Navigation Guided Surgery