J Periodontal Implant Sci.
2019 Oct;49(5):330-343. 10.5051/jpis.2019.49.5.330.
Comparative preclinical assessment of the use of dehydrated human amnion/chorion membrane to repair perforated sinus membranes
- Affiliations
-
- 1Department of Dentistry, Inha International Medical Center, Incheon, Korea.
- 2Department of Periodontics, Asan Medical Center & Department of Dentistry, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Periodontology, College of Dentistry and Institute of Oral Bioscience, Chonbuk National University, Jeonju, Korea. grayheron@hanmail.net
- 4Research Institute of Clinical Medicine of Chonbuk National University-Biomedical Research Institute of Chonbuk National University Hospital, Jeonju, Korea.
- KMID: 2461023
- DOI: http://doi.org/10.5051/jpis.2019.49.5.330
Abstract
- PURPOSE
The aim of this study was to evaluate the use of dehydrated human amnion/chorion membrane (dHACM) to repair perforated sinus membranes in rabbits.
METHODS
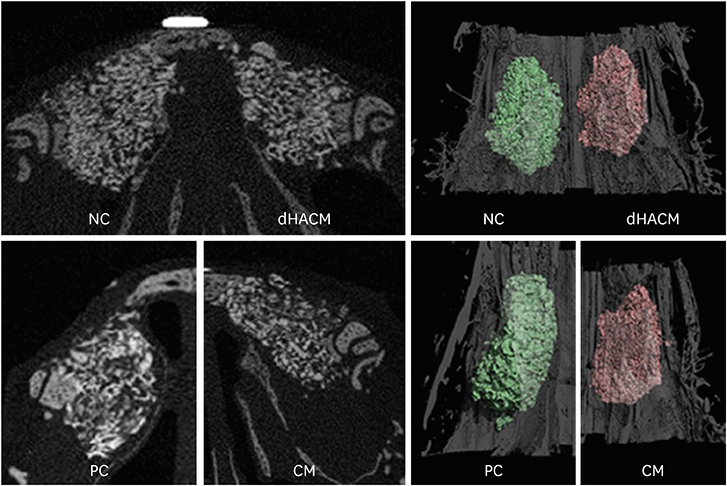
Bilateral surgical windows (7.5-mm diameter) were prepared on the nasal bones of 14 rabbits. Standardized circular perforations (5-mm diameter) were made in the sinus membrane by manipulating implant twist drills. The perforated sinus membranes were repaired using dHACM or a resorbable collagen membrane (CM). The negative control (NC) group did not undergo perforated sinus membrane repair, while the positive control (PC) group underwent sinus augmentation without perforations. The same amount of deproteinized porcine bone mineral was grafted in all 4 groups. After 6 weeks, micro-computed tomography (micro-CT) and histomorphometric evaluations were conducted.
RESULTS
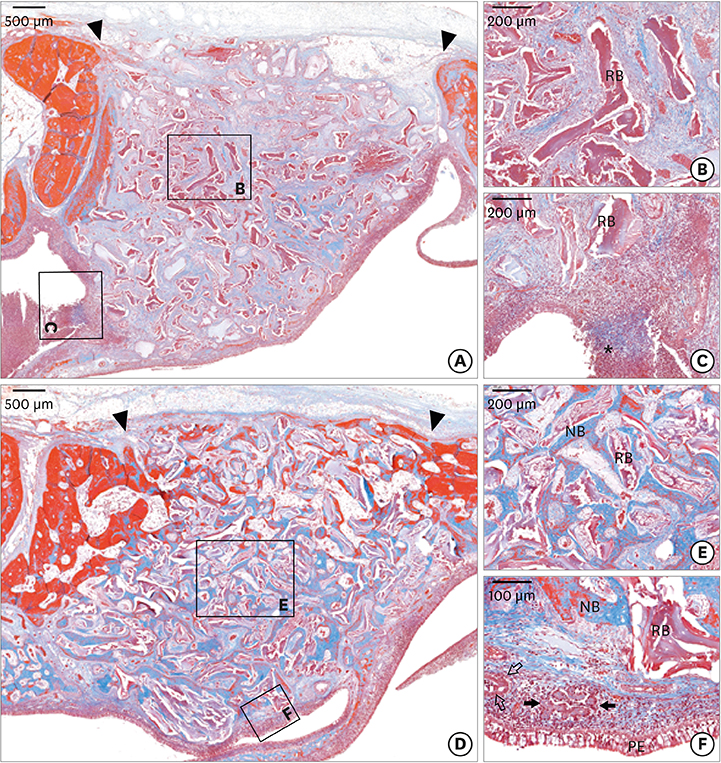
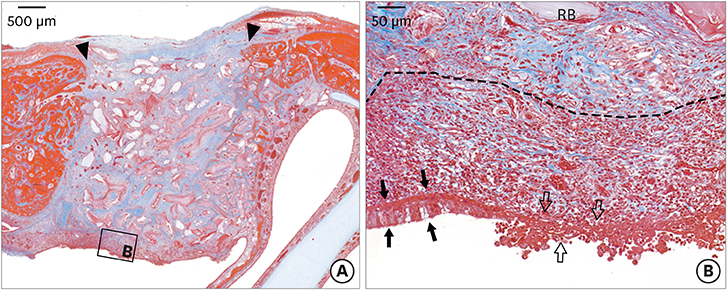
The micro-CT analysis revealed that the total augmented volume was not significantly different among the groups. In the dHACM group, newly formed bone filled the augmented area with remaining biomaterials; however, non-ciliated flat epithelium and inflammatory cells were observed on the healed sinus membrane. Histometric analysis showed that the percentage of newly formed bone area in the dHACM group did not differ significantly from that in the CM group. The dHACM group showed a significantly higher percentage of newly formed bone area than the NC group, but there was no significant difference between the dHACM and PC groups.
CONCLUSIONS
dHACM could be a feasible solution for repairing sinus membrane perforations that occur during sinus floor augmentation.
Keyword
MeSH Terms
Figure
Reference
-
1. Schwartz-Arad D, Herzberg R, Dolev E. The prevalence of surgical complications of the sinus graft procedure and their impact on implant survival. J Periodontol. 2004; 75:511–516.
Article2. Hernández-Alfaro F, Torradeflot MM, Marti C. Prevalence and management of Schneiderian membrane perforations during sinus-lift procedures. Clin Oral Implants Res. 2008; 19:91–98.
Article3. Stacchi C, Andolsek F, Berton F, Perinetti G, Navarra CO, Di Lenarda R. Intraoperative complications during sinus floor elevation with lateral approach: a systematic review. Int J Oral Maxillofac Implants. 2017; 32:e107–e118.
Article4. de Almeida Ferreira CE, Martinelli CB, Novaes AB Jr, Pignaton TB, Guignone CC, Gonçalves de Almeida AL, et al. Effect of maxillary sinus membrane perforation on implant survival rate: a retrospective study. Int J Oral Maxillofac Implants. 2017; 32:401–407.
Article5. Nolan PJ, Freeman K, Kraut RA. Correlation between Schneiderian membrane perforation and sinus lift graft outcome: a retrospective evaluation of 359 augmented sinus. J Oral Maxillofac Surg. 2014; 72:47–52.
Article6. Proussaefs P, Lozada J, Kim J, Rohrer MD. Repair of the perforated sinus membrane with a resorbable collagen membrane: a human study. Int J Oral Maxillofac Implants. 2004; 19:413–420.7. Testori T, Wallace SS, Del Fabbro M, Taschieri S, Trisi P, Capelli M, et al. Repair of large sinus membrane perforations using stabilized collagen barrier membranes: surgical techniques with histologic and radiographic evidence of success. Int J Periodontics Restorative Dent. 2008; 28:9–17.8. Becker ST, Terheyden H, Steinriede A, Behrens E, Springer I, Wiltfang J. Prospective observation of 41 perforations of the Schneiderian membrane during sinus floor elevation. Clin Oral Implants Res. 2008; 19:1285–1289.
Article9. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008; 15:88–99.
Article10. Koob TJ, Lim JJ, Massee M, Zabek N, Denozière G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater. 2014; 102:1353–1362.
Article11. Kesting MR, Wolff KD, Nobis CP, Rohleder NH. Amniotic membrane in oral and maxillofacial surgery. Oral Maxillofac Surg. 2014; 18:153–164.
Article12. Koob TJ, Lim JJ, Massee M, Zabek N, Rennert R, Gurtner G, et al. Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell. 2014; 6:10.
Article13. Hashim SN, Yusof MF, Zahari W, Noordin KB, Kannan TP, Hamid SS, et al. Angiogenic potential of extracellular matrix of human amniotic membrane. Tissue Eng Regen Med. 2016; 13:211–217.
Article14. Arai N, Tsuno H, Okabe M, Yoshida T, Koike C, Noguchi M, et al. Clinical application of a hyperdry amniotic membrane on surgical defects of the oral mucosa. J Oral Maxillofac Surg. 2012; 70:2221–2228.
Article15. Rosen PS, Froum SJ, Cohen DW. Consecutive case series using a composite allograft containing mesenchymal cells with an amnion-chorion barrier to treat mandibular Class III/IV furcations. Int J Periodontics Restorative Dent. 2015; 35:453–460.
Article16. Velez I, Parker WB, Siegel MA, Hernandez M. Cryopreserved amniotic membrane for modulation of periodontal soft tissue healing: a pilot study. J Periodontol. 2010; 81:1797–1804.
Article17. Kothari CR, Goudar G, Hallur N, Sikkerimath B, Gudi S, Kothari MC. Use of amnion as a graft material in vestibuloplasty: a clinical study. Br J Oral Maxillofac Surg. 2012; 50:545–549.
Article18. Shah R, Sowmya NK, Mehta DS. Amnion membrane for coverage of gingival recession: a novel application. Contemp Clin Dent. 2014; 5:293–295.
Article19. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother. 2010; 1:94–99.
Article20. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007; 39:175–191.
Article21. Yon J, Lee JS, Lim HC, Kim MS, Hong JY, Choi SH, et al. Pre-clinical evaluation of the osteogenic potential of bone morphogenetic protein-2 loaded onto a particulate porcine bone biomaterial. J Clin Periodontol. 2015; 42:81–88.
Article22. Kang H. Random allocation and dynamic allocation randomization. Anesth Pain Med. 2017; 12:201–212.
Article23. Moon YS, Sohn DS, Moon JW, Lee JH, Park IS, Lee JK. Comparative histomorphometric analysis of maxillary sinus augmentation with absorbable collagen membrane and osteoinductive replaceable bony window in rabbits. Implant Dent. 2014; 23:29–36.
Article24. Choi Y, Yun JH, Kim CS, Choi SH, Chai JK, Jung UW. Sinus augmentation using absorbable collagen sponge loaded with Escherichia coli-expressed recombinant human bone morphogenetic protein 2 in a standardized rabbit sinus model: a radiographic and histologic analysis. Clin Oral Implants Res. 2012; 23:682–689.
Article25. Lim HC, Hong JY, Lee JS, Jung UW, Choi SH. Late-term healing in an augmented sinus with different ratios of biphasic calcium phosphate: a pilot study using a rabbit sinus model. J Periodontal Implant Sci. 2016; 46:57–69.
Article26. Holtzclaw D. Maxillary sinus membrane repair with amnion-chorion barriers: a retrospective case series. J Periodontol. 2015; 86:936–940.
Article27. Lee JS, Shin HK, Yun JH, Cho KS. Randomized clinical trial of maxillary sinus grafting using deproteinized porcine and bovine bone mineral. Clin Implant Dent Relat Res. 2017; 19:140–150.
Article28. Forsgren K, Stierna P, Kumlien J, Carlsöö B. Regeneration of maxillary sinus mucosa following surgical removal. Experimental study in rabbits. Ann Otol Rhinol Laryngol. 1993; 102:459–466.
Article29. Watanabe K, Niimi A, Ueda M. Autogenous bone grafts in the rabbit maxillary sinus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 88:26–32.
Article30. Al-Moraissi E, Elsharkawy A, Abotaleb B, Alkebsi K, Al-Motwakel H. Does intraoperative perforation of Schneiderian membrane during sinus lift surgery causes an increased the risk of implants failure?: a systematic review and meta regression analysis. Clin Implant Dent Relat Res. 2018; 20:882–889.
Article31. Lim HC, Son Y, Hong JY, Shin SI, Jung UW, Chung JH. Sinus floor elevation in sites with a perforated schneiderian membrane: What is the effect of placing a collagen membrane in a rabbit model? Clin Oral Implants Res. 2018; 29:1202–1211.
Article32. Favero V, Lang NP, Canullo L, Urbizo Velez J, Bengazi F, Botticelli D. Sinus floor elevation outcomes following perforation of the Schneiderian membrane. An experimental study in sheep. Clin Oral Implants Res. 2016; 27:233–240.
Article33. Benninger MS, Schmidt JL, Crissman JD, Gottlieb C. Mucociliary function following sinus mucosal regeneration. Otolaryngol Head Neck Surg. 1991; 105:641–648.
Article34. Min YG, Kim IT, Park SH. Mucociliary activity and ultrastructural abnormalities of regenerated sinus mucosa in rabbits. Laryngoscope. 1994; 104:1482–1486.
Article35. Erickson VR, Antunes M, Chen B, Cohen NA, Hwang PH. The effects of retinoic acid on ciliary function of regenerated sinus mucosa. Am J Rhinol. 2008; 22:334–336.
Article36. Kim YM, Lee CH, Won TB, Kim SW, Kim JW, Rhee CS, et al. Functional recovery of rabbit maxillary sinus mucosa in two different experimental injury models. Laryngoscope. 2008; 118:541–545.
Article37. Froum SJ, Khouly I, Favero G, Cho SC. Effect of maxillary sinus membrane perforation on vital bone formation and implant survival: a retrospective study. J Periodontol. 2013; 84:1094–1099.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Repair of an oroantral communication by a human amniotic membrane: a novel technique
- Repair of the perforated sinus membrane with a micro-suture technique : Report of cases
- Changes in the Expression of Cyclooxygenase-2 after Labor in Myometrium and Fetal Membrane of Term Pregnancy
- Subcellular localization of nuclear factor kappa B in term human fetal membranes and myometrium during labor
- Intra-sinus rigid fixation of a resorbable barrier membrane to repair a large perforation of the sinus membrane: a technical note