Korean J Ophthalmol.
2019 Oct;33(5):414-421. 10.3341/kjo.2019.0057.
Effect and Mechanism of Phosphodiesterase Inhibitors on Trabecular Outflow
- Affiliations
-
- 1Department of Ophthalmology, Daegu Catholic University School of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2459522
- DOI: http://doi.org/10.3341/kjo.2019.0057
Abstract
- PURPOSE
Phosphodiesterase (PDE) inhibitors increase matrix metalloproteinase (MMP) production by inhibiting re-uptake of adenosine and may potentiate nitric oxide (NO) activity. This study was performed to investigate the effects and mechanisms of PDE inhibitors on trabecular outflow in cultured human trabecular meshwork cells (HTMCs).
METHODS
Primary HTMC cultures were exposed to 0, 20, and 50 µM dipyridamole (DPD) or theophylline (TPN). Permeability through the HTMC monolayer was assessed using carboxyfluorescein. The production of NO was assessed using the Griess assay and MMP-2 levels were measured via Western blotting.
RESULTS
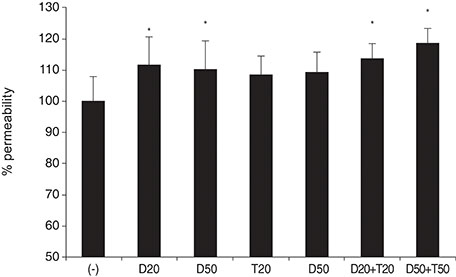
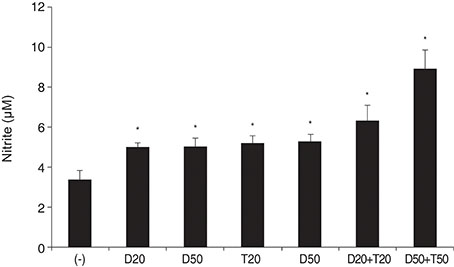
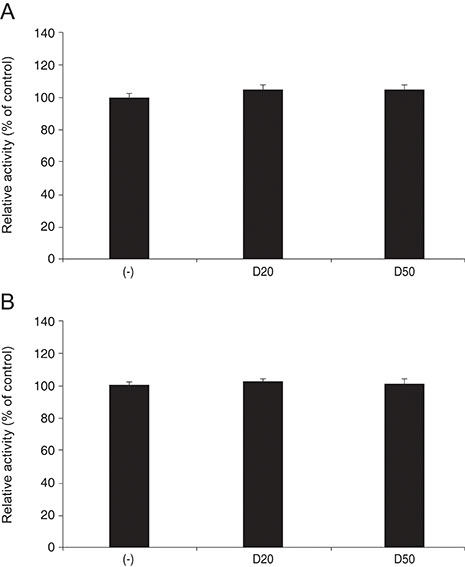
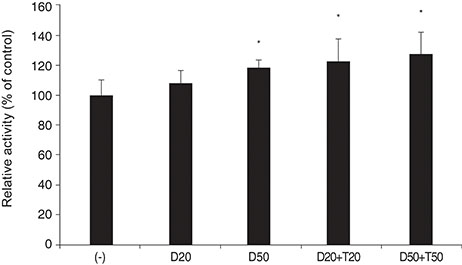
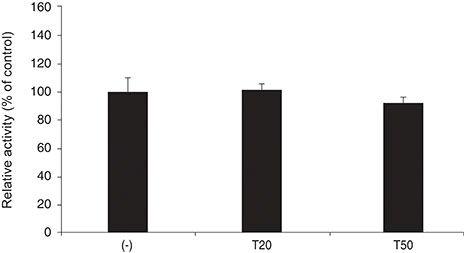
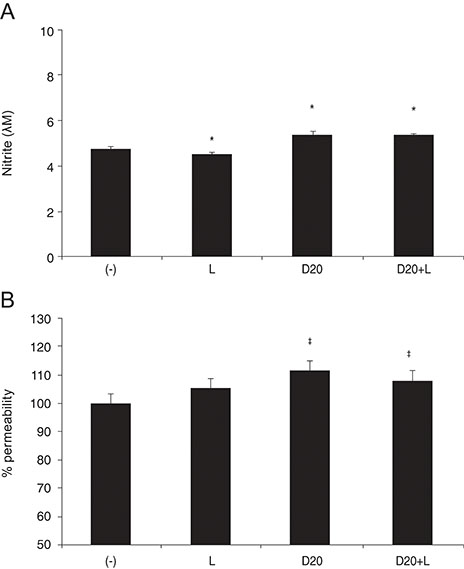
DPD significantly increased permeability accompanied with increased nitrite concentration and MMP-2 levels (all p < 0.05). TPN increased nitrite but did not affect permeability or MMP-2 levels significantly (p > 0.05). When treated with DPD and TPN together, both permeability and nitrite production were increased; however, MMP-2 levels showed no difference compared to DPD exposure alone (p > 0.05).
CONCLUSIONS
DPD increased trabecular permeability accompanied with increased nitrite production and MMP-2 levels. PDE inhibitors may increase trabecular outflow by increasing MMP-2 levels and by potentiating NO activity through cyclic GMP in HTMC.
MeSH Terms
Figure
Reference
-
1. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–579.
Article2. Rohen JW, Lutjen-Drecoll E, Flugel C, et al. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG). Exp Eye Res. 1993; 56:683–692.3. Schmidl D, Schmetterer L, Garhofer G, Popa-Cherecheanu A. Pharmacotherapy of glaucoma. J Ocul Pharmacol Ther. 2015; 31:63–77.
Article4. Kopczynski CC, Epstein DL. Emerging trabecular outflow drugs. J Ocul Pharmacol Ther. 2014; 30:85–87.
Article5. Gao ZG, Jacobson KA. Emerging adenosine receptor agonists: an update. Expert Opin Emerg Drugs. 2011; 16:597–602.6. Sanka K, Maddala R, Epstein DL, Rao PV. Influence of actin cytoskeletal integrity on matrix metalloproteinase-2 activation in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007; 48:2105–2114.
Article7. Shearer TW, Crosson CE. Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002; 43:3016–3020.8. Gamboa A, Abraham R, Diedrich A, et al. Role of adenosine and nitric oxide on the mechanisms of action of dipyridamole. Stroke. 2005; 36:2170–2175.
Article9. Sansone GR, Matin A, Wang SF, et al. Theophylline inhibits the production of nitric oxide by peripheral blood mononuclear cells from patients with asthma. Ann Allergy Asthma Immunol. 1998; 81:90–95.
Article10. Venkatesh PK, Pattillo CB, Branch B, et al. Dipyridamole enhances ischaemia-induced arteriogenesis through an endocrine nitrite/nitric oxide-dependent pathway. Cardiovasc Res. 2010; 85:661–670.
Article11. Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994; 35:2515–2520.12. Behar-Cohen FF, Goureau O, D'Hermies F, Courtois Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest Ophthalmol Vis Sci. 1996; 37:1711–1715.13. Grimes PA, Stone RA, Laties AM, Li W. Carboxyfluorescein. A probe of the blood-ocular barriers with lower membrane permeability than f luorescein. Arch Ophthalmol. 1982; 100:635–639.14. Araie M. Carboxyfluorescein: a dye for evaluating the corneal endothelial barrier function in vivo. Exp Eye Res. 1986; 42:141–150.
Article15. Grimes PA. Carboxyf luorescein transfer across the blood-retinal barrier evaluated by quantitative fluorescence microscopy: comparison with fluorescein. Exp Eye Res. 1988; 46:769–783.16. Nakagawa S, Usui T, Yokoo S, et al. Toxicity evaluation of antiglaucoma drugs using stratified human cultivated corneal epithelial sheets. Invest Ophthalmol Vis Sci. 2012; 53:5154–5160.
Article17. Lei Y, Stamer WD, Wu J, Sun X. Oxidative stress impact on barrier function of porcine angular aqueous plexus cell monolayers. Invest Ophthalmol Vis Sci. 2013; 54:4827–4835.
Article18. Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–138.
Article19. Zhong Y, Yang Z, Huang WC, Luo X. Adenosine, adenosine receptors and glaucoma: an updated overview. Biochim Biophys Acta. 2013; 1830:2882–2890.
Article20. Crosson CE. Adenosine receptor activation modulates intraocular pressure in rabbits. J Pharmacol Exp Ther. 1995; 273:320–326.21. Tian B, Gabelt BT, Crosson CE, Kaufman PL. Effects of adenosine agonists on intraocular pressure and aqueous humor dynamics in cynomolgus monkeys. Exp Eye Res. 1997; 64:979–989.
Article22. Fleischhauer JC, Mitchell CH, Stamer WD, et al. Common actions of adenosine receptor agonists in modulating human trabecular meshwork cell transport. J Membr Biol. 2003; 193:121–136.
Article23. Crosson CE. Intraocular pressure responses to the adenosine agonist cyclohexyladenosine: evidence for a dual mechanism of action. Invest Ophthalmol Vis Sci. 2001; 42:1837–1840.24. Li A, Leung CT, Peterson-Yantorno K, et al. Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow. J Cell Physiol. 2012; 227:172–182.
Article25. Sanderson J, Dartt DA, Trinkaus-Randall V, et al. Purines in the eye: recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Muller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp Eye Res. 2014; 127:270–279.26. Kim JW. Comparative study of the effects of trabecular meshwork outflow drugs on the permeability and nitric oxide production in trabecular meshwork cells. Korean J Ophthalmol. 2017; 31:452–459.
Article27. Barnes PJ. Theophylline. Am J Respir Crit Care Med. 2013; 188:901–906.
Article28. Chen HH, Wang DL. Nitric oxide inhibits matrix metalloproteinase-2 expression via the induction of activating transcription factor 3 in endothelial cells. Mol Pharmacol. 2004; 65:1130–1140.
Article29. Kim JW. Effect of nitric oxide on the expression of matrix metalloproteinase and its association with migration of cultured trabecular meshwork cells. Korean J Ophthalmol. 2016; 30:66–75.
Article30. Keller KE, Aga M, Bradley JM, et al. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009; 88:676–682.
Article31. Bradley JM, Vranka J, Colvis CM, et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998; 39:2649–2658.32. Pang IH, Hellberg PE, Fleenor DL, et al. Expression of matrix metalloproteinases and their inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003; 44:3485–3493.
Article33. Li A, Leung CT, Peterson-Yantorno K, et al. Cytoskeletal dependence of adenosine triphosphate release by human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011; 52:7996–8005.
Article34. Baek MJ, Kim KH, Kim JW. Regulation of matrix metalloproteinase 2 expression by an adenosine A1 agonist in trabecular meshwork cells. J Korean Ophthalmol Soc. 2018; 59:946–952.
Article35. Guo S, Stins M, Ning M, Lo EH. Amelioration of inflammation and cytotoxicity by dipyridamole in brain endothelial cells. Cerebrovasc Dis. 2010; 30:290–296.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytoprotective Effect of Rho Kinase Inhibitors against Oxidative Stress inTrabecular Meshwork Cells
- The Effect of Interleukin-1alpha on Trabecular Outflow Resistance in Rat Eyes
- Effect of Bimatoprost on the Permeability of Trabecular Meshwork Cell Monolayer
- Chronic Low Dosing of Phosphodiesterase Type 5 Inhibitor for Erectile Dysfunction
- Treatment Strategy for Non-Responders to PDE5 Inhibitors