J Gastric Cancer.
2019 Mar;19(1):121-131. 10.5230/jgc.2019.19.e9.
Prognostic Threshold of Neuroendocrine Differentiation in Gastric Carcinoma: a Clinicopathological Study of 945 Cases
- Affiliations
-
- 1Department of Pathology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China. zhgshg@126.com
- 2Department of Pathology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China.
- 3Public Security Bureau of Changle City, Changle, China.
- 4Department of Pathology, No. 2 Hospital, Xiamen, China.
- 5Department of Pathology, Fujian Provincial Cancer Hospital, Fuzhou, China.
- 6Maternity and Child Care Hospital of Huli District, Xiamen, China.
- KMID: 2458824
- DOI: http://doi.org/10.5230/jgc.2019.19.e9
Abstract
- PURPOSE
The significance of neuroendocrine differentiation (NED) in gastric carcinoma (GC) is controversial, leading to ambiguous concepts in traditional classifications. This study aimed to determine the prognostic threshold of meaningful NED in GC and clarify its unclear features in existing classifications.
MATERIALS AND METHODS
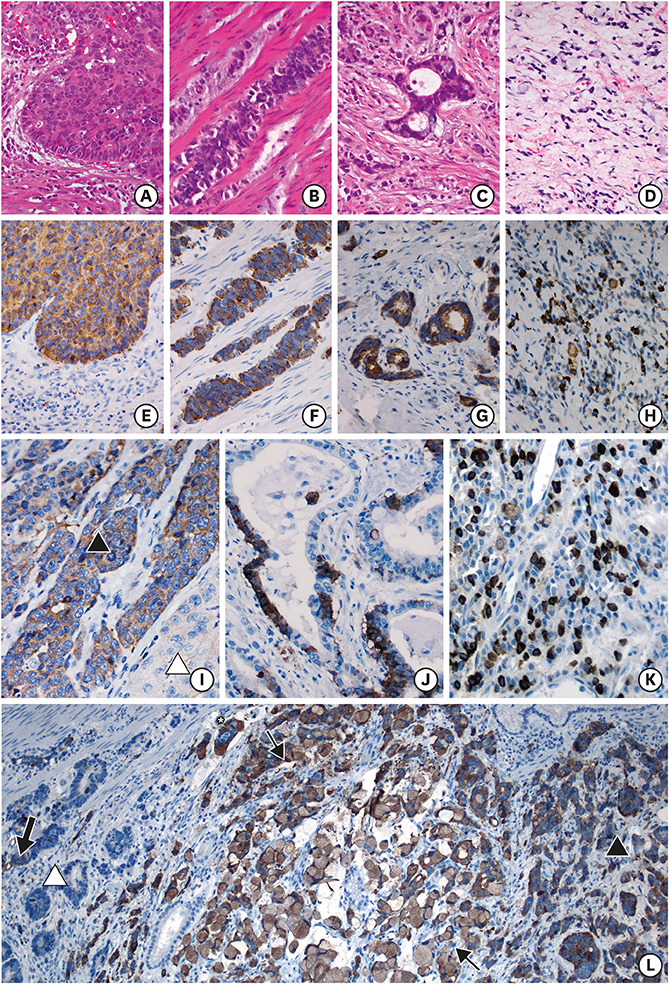
Immunohistochemical staining for synaptophysin, chromogranin A, and neural cell adhesion molecule was performed for 945 GC specimens. Survival analysis was performed using the log-rank test and univariate/multivariate models with percentages of NED (PNED) and demographic and clinicopathological parameters.
RESULTS
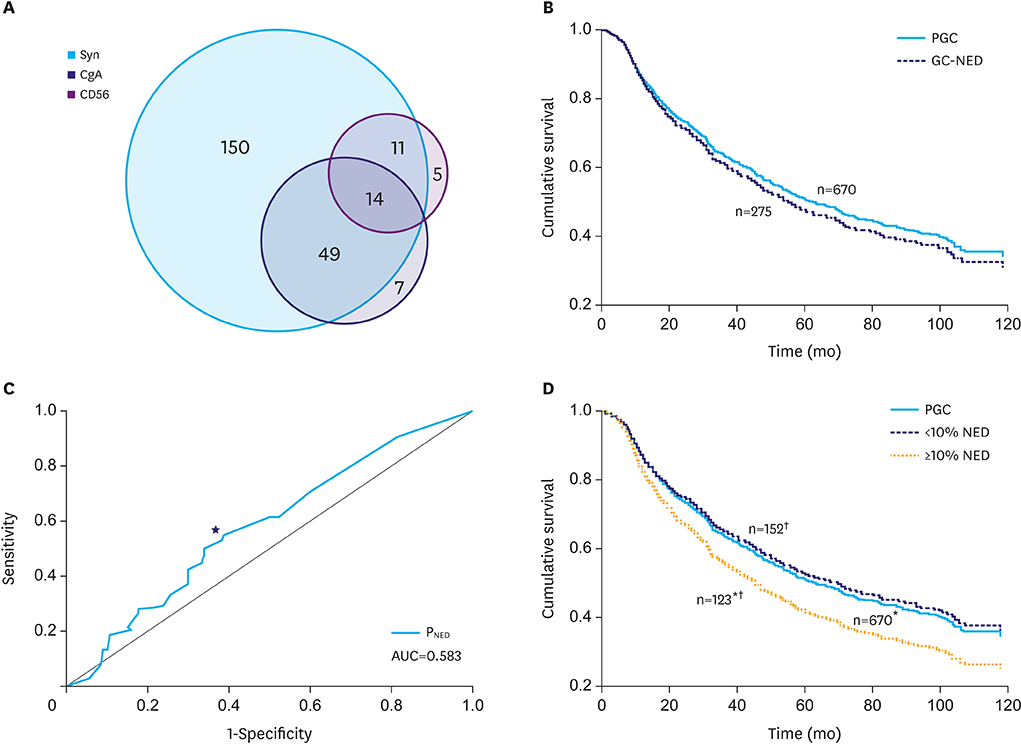
In total, 275 (29.1%) cases were immunoreactive to at least 1 neuroendocrine (NE) marker. GC-NED was more common in the upper third of the stomach. PNED, and Borrmann's classification and tumor, lymph node, metastasis stages were independent prognostic factors. The cutoff PNED was 10%, beyond which patients had significantly worse outcomes, although the risk did not increase with higher PNED. Tumors with ≥10% NED tended to manifest as Borrmann type III lesion with mixed/diffuse morphology and poorer histological differentiation; the NE components in this population mainly grew in insulae/nests, which differed from the predominant growth pattern (glandular/acinar) in GC with <10% NED.
CONCLUSIONS
GC with ≥10% NED should be classified as a distinct subtype because of its worse prognosis, and more attention should be paid to the necessity of additional therapeutics for NE components.
MeSH Terms
Figure
Reference
-
1. Ooi A, Mai M, Ogino T, Ueda H, Kitamura T, Takahashi Y, et al. Endocrine differentiation of gastric adenocarcinoma. The prevalence as evaluated by immunoreactive chromogranin A and its biologic significance. Cancer. 1988; 62:1096–1104.
Article2. Qvigstad G, Sandvik AK, Brenna E, Aase S, Waldum HL. Detection of chromogranin A in human gastric adenocarcinomas using a sensitive immunohistochemical technique. Histochem J. 2000; 32:551–556.3. Sentani K, Oue N, Noguchi T, Sakamoto N, Matsusaki K, Yasui W. Immunostaining of gastric cancer with neuroendocrine differentiation: Reg IV-positive neuroendocrine cells are associated with gastrin, serotonin, pancreatic polypeptide and somatostatin. Pathol Int. 2010; 60:291–297.
Article4. Jiang SX, Mikami T, Umezawa A, Saegusa M, Kameya T, Okayasu I. Gastric large cell neuroendocrine carcinomas: a distinct clinicopathologic entity. Am J Surg Pathol. 2006; 30:945–953.
Article5. Canzonieri V, Colarossi C, Del Col L, Perin T, Talamini R, Sigon R, et al. Exocrine and endocrine modulation in common gastric carcinoma. Am J Clin Pathol. 2012; 137:712–721.
Article6. Eren F, Celikel C, Güllüoğlu B. Neuroendocrine differentiation in gastric adenocarcinomas; correlation with tumor stage and expression of VEGF and p53. Pathol Oncol Res. 2004; 10:47–51.
Article7. Blumenfeld W, Chandhoke DK, Sagerman P, Turi GK. Neuroendocrine differentiation in gastric adenocarcinomas. An immunohistochemical study. Arch Pathol Lab Med. 1996; 120:478–481.8. Waldum HL, Aase S, Kvetnoi I, Brenna E, Sandvik AK, Syversen U, et al. Neuroendocrine differentiation in human gastric carcinoma. Cancer. 1998; 83:435–444.
Article9. Fujii A, Kamiakito T, Takayashiki N, Fujii T, Tanaka A. Neuroendocrine tissue-specific transcription factor, BETA2/NeuroD, in gastric carcinomas: a comparison with chromogranin A and synaptophysin expressions. Pathol Res Pract. 2003; 199:513–519.
Article10. Wang LL, Yao GY, Zhao ZS, Wei XL, Xu RJ. Clonality analysis of neuroendocrine cells in gastric adenocarcinoma. World J Gastroenterol. 2013; 19:5340–5346.
Article11. Yao GY, Zhou JL, Lai MD, Chen XQ, Chen PH. Neuroendocrine markers in adenocarcinomas: an investigation of 356 cases. World J Gastroenterol. 2003; 9:858–861.
Article12. Zhang T, Su D, Mao Z, Guo X, Wang L, Bai L. Prognostic role of neuroendocrine cell differentiation in human gastric carcinoma. Int J Clin Exp Med. 2015; 8:7837–7842.13. Kim JJ, Kim JY, Hur H, Cho YK, Han SU. Clinicopathologic significance of gastric adenocarcinoma with neuroendocrine features. J Gastric Cancer. 2011; 11:195–199.
Article14. Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer;2010.15. Park JY, Ryu MH, Park YS, Park HJ, Ryoo BY, Kim MG, et al. Prognostic significance of neuroendocrine components in gastric carcinomas. Eur J Cancer. 2014; 50:2802–2809.
Article16. Kim TY, Chae HD. Composite neuroendocrine carcinoma with adenocarcinoma of the stomach misdiagnosed as a giant submucosal tumor. J Gastric Cancer. 2011; 11:126–130.
Article17. Granberg D, Wilander E, Stridsberg M, Granerus G, Skogseid B, Oberg K. Clinical symptoms, hormone profiles, treatment, and prognosis in patients with gastric carcinoids. Gut. 1998; 43:223–228.
Article18. Volante M, Rindi G, Papotti M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006; 449:499–506.
Article19. Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987; 11:Suppl 1. 71–86.
Article20. Chang S, Choi D, Lee SJ, Lee WJ, Park MH, Kim SW, et al. Neuroendocrine neoplasms of the gastrointestinal tract: classification, pathologic basis, and imaging features. Radiographics. 2007; 27:1667–1679.
Article21. Leoncini E, Carioli G, La Vecchia C, Boccia S, Rindi G. Risk factors for neuroendocrine neoplasms: a systematic review and meta-analysis. Ann Oncol. 2016; 27:68–81.
Article22. Gill S, Shah A, Le N, Cook EF, Yoshida EM. Asian ethnicity-related differences in gastric cancer presentation and outcome among patients treated at a Canadian cancer center. J Clin Oncol. 2003; 21:2070–2076.
Article23. Ng CJ, Teo CH, Abdullah N, Tan WP, Tan HM. Relationships between cancer pattern, country income and geographical region in Asia. BMC Cancer. 2015; 15:613.
Article24. Tanaka T, Kaneko M, Nozawa H, Emoto S, Murono K, Otani K, et al. Diagnosis, assessment, and therapeutic strategy for colorectal mixed adenoneuroendocrine carcinoma. Neuroendocrinology. 2017; 105:426–434.
Article25. Chen H, Shu M, Chen S, Xue L, Lin Y. Clinicopathological features and lymph node metastatic patterns of gastric mixed adenoneuroendocrine carcinoma. Histol Histopathol. 2018.26. Xie JW, Lu J, Wang JB, Lin JX, Chen QY, Cao LL, et al. Prognostic factors for survival after curative resection of gastric mixed adenoneuroendocrine carcinoma: a series of 80 patients. BMC Cancer. 2018; 18:1021.
Article27. Yang G, Li D, Zheng F, Yang L. Long-term disease free survival of gastric mixed adenoneuroendocrine carcinoma treated with multimodality therapy: a case report. Mol Clin Oncol. 2018; 8:653–656.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prognostic Significance of Neuroendocrine Differentiation for Treating Prostatic Carcinoma in 699 Cases of Radical Prostatectomy
- Large Cell Neuroendocrine Carcinoma of the Lung: Report of three cases
- Diagnosis and Treatment of Gastric Neuroendocrine Tumors
- Gastric Collision Tumor (Adenocarcinoma and Neuro-endocrine Carcinoma): A Report of Two Cases
- Gastric Adenocarcinoma with Coexistent Hepatoid Adenocarcinoma and Neuroendocrine Carcinoma: A Case Report