Korean J Radiol.
2016 Oct;17(5):633-640. 10.3348/kjr.2016.17.5.633.
A Comparison of Substantia Nigra T1 Hyperintensity in Parkinson's Disease Dementia, Alzheimer's Disease and Age-Matched Controls: Volumetric Analysis of Neuromelanin Imaging
- Affiliations
-
- 1Department of Radiology, Konkuk University School of Medicine, Seoul 05030, Korea. mdmoonwj@kuh.ac.kr
- 2Department of Radiology, Asan Medical Center, Seoul 05505, Korea.
- 3Department of Neurology, Konkuk University School of Medicine, Seoul 05030, Korea.
- 4Department of Biomedical Engineering, Hanyang University, Seoul 04763, Korea.
- KMID: 2458054
- DOI: http://doi.org/10.3348/kjr.2016.17.5.633
Abstract
OBJECTIVE
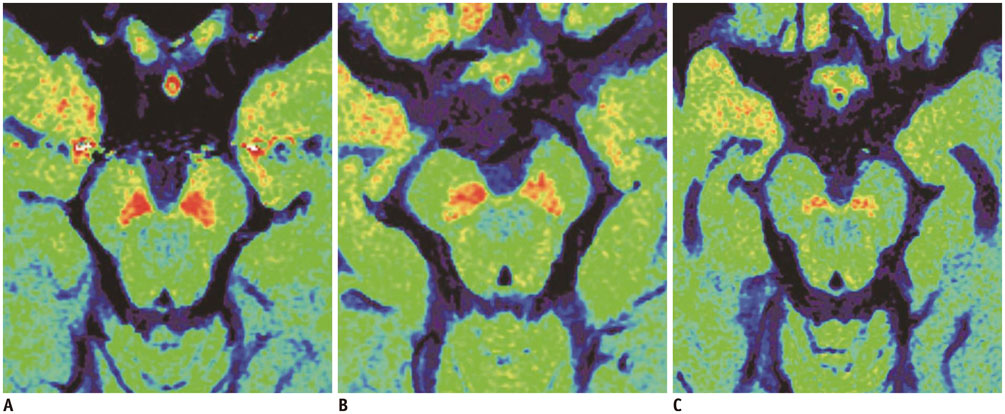
Neuromelanin loss of substantia nigra (SN) can be visualized as a T1 signal reduction on T1-weighted high-resolution imaging. We investigated whether volumetric analysis of T1 hyperintensity for SN could be used to differentiate between Parkinson's disease dementia (PDD), Alzheimer's disease (AD) and age-matched controls.
MATERIALS AND METHODS
This retrospective study enrolled 10 patients with PDD, 18 patients with AD, and 13 age-matched healthy elderly controls. MR imaging was performed at 3 tesla. To measure the T1 hyperintense area of SN, we obtained an axial thin section high-resolution T1-weighted fast spin echo sequence. The volumes of interest for the T1 hyperintense SN were drawn onto heavily T1-weighted FSE sequences through midbrain level, using the MIPAV software. The measurement differences were tested using the Kruskal-Wallis test followed by a post hoc comparison.
RESULTS
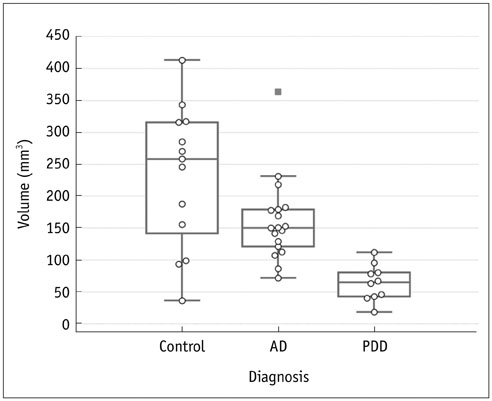
A comparison of the three groups showed significant differences in terms of volume of T1 hyperintensity (p < 0.001, Bonferroni corrected). The volume of T1 hyperintensity was significantly lower in PDD than in AD and normal controls (p < 0.005, Bonferroni corrected). However, the volume of T1 hyperintensity was not different between AD and normal controls (p = 0.136, Bonferroni corrected).
CONCLUSION
The volumetric measurement of the T1 hyperintensity of SN can be an imaging marker for evaluating neuromelanin loss in neurodegenerative diseases and a differential in PDD and AD cases.
Keyword
MeSH Terms
-
Aged
Aged, 80 and over
Alzheimer Disease/diagnostic imaging
Biomarkers/metabolism
Dementia/*diagnostic imaging/etiology
Diagnosis, Differential
Female
Humans
Magnetic Resonance Imaging/methods
Male
Melanins/*metabolism
Middle Aged
Observer Variation
Parkinson Disease/*diagnostic imaging/psychology
Retrospective Studies
Software
Substantia Nigra/*diagnostic imaging/metabolism
Biomarkers
Melanins
Figure
Reference
-
1. Zecca L, Tampellini D, Gerlach M, Riederer P, Fariello RG, Sulzer D. Substantia nigra neuromelanin: structure, synthesis, and molecular behaviour. Mol Pathol. 2001; 54:414–418.2. Double KL, Ben-Shachar D, Youdim MB, Zecca L, Riederer P, Gerlach M. Influence of neuromelanin on oxidative pathways within the human substantia nigra. Neurotoxicol Teratol. 2002; 24:621–628.3. Zecca L, Zucca FA, Wilms H, Sulzer D. Neuromelanin of the substantia nigra: a neuronal black hole with protective and toxic characteristics. Trends Neurosci. 2003; 26:578–580.4. Sasaki M, Shibata E, Tohyama K, Takahashi J, Otsuka K, Tsuchiya K, et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease. Neuroreport. 2006; 17:1215–1218.5. Sasaki M, Shibata E, Tohyama K, Kudo K, Endoh J, Otsuka K, et al. Monoamine neurons in the human brain stem: anatomy, magnetic resonance imaging findings, and clinical implications. Neuroreport. 2008; 19:1649–1654.6. Matsuura K, Maeda M, Yata K, Ichiba Y, Yamaguchi T, Kanamaru K, et al. Neuromelanin magnetic resonance imaging in Parkinson's disease and multiple system atrophy. Eur Neurol. 2013; 70:70–77.7. Ohtsuka C, Sasaki M, Konno K, Kato K, Takahashi J, Yamashita F, et al. Differentiation of early-stage parkinsonisms using neuromelanin-sensitive magnetic resonance imaging. Parkinsonism Relat Disord. 2014; 20:755–760.8. Kashihara K, Shinya T, Higaki F. Reduction of neuromelanin-positive nigral volume in patients with MSA, PSP and CBD. Intern Med. 2011; 50:1683–1687.9. Shibata E, Sasaki M, Tohyama K, Otsuka K, Endoh J, Terayama Y, et al. Use of neuromelanin-sensitive MRI to distinguish schizophrenic and depressive patients and healthy individuals based on signal alterations in the substantia nigra and locus ceruleus. Biol Psychiatry. 2008; 64:401–406.10. Song IU, Kim JS, Yoo JY, Song HJ, Lee KS. Cognitive dysfunctions in mild Parkinson's disease dementia: comparison with patients having mild Alzheimer's disease and normal controls. Eur Neurol. 2008; 59:49–54.11. Song IU, Chung YA, Chung SW, Jeong J. Early diagnosis of Alzheimer's disease and Parkinson's disease associated with dementia using cerebral perfusion SPECT. Dement Geriatr Cogn Disord. 2014; 37:276–285.12. Martorana A, Koch G. "Is dopamine involved in Alzheimer's disease?". Front Aging Neurosci. 2014; 6:252.13. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992; 55:181–184.14. Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007; 22:1689–1707.15. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984; 34:939–944.16. Kitao S, Matsusue E, Fujii S, Miyoshi F, Kaminou T, Kato S, et al. Correlation between pathology and neuromelanin MR imaging in Parkinson's disease and dementia with Lewy bodies. Neuroradiology. 2013; 55:947–953.17. Kashihara K, Shinya T, Higaki F. Neuromelanin magnetic resonance imaging of nigral volume loss in patients with Parkinson's disease. J Clin Neurosci. 2011; 18:1093–1096.18. Schwarz ST, Rittman T, Gontu V, Morgan PS, Bajaj N, Auer DP. T1-weighted MRI shows stage-dependent substantia nigra signal loss in Parkinson's disease. Mov Disord. 2011; 26:1633–1638.19. Miyoshi F, Ogawa T, Kitao SI, Kitayama M, Shinohara Y, Takasugi M, et al. Evaluation of Parkinson disease and Alzheimer disease with the use of neuromelanin MR imaging and (123)I-metaiodobenzylguanidine scintigraphy. AJNR Am J Neuroradiol. 2013; 34:2113–2118.20. Ogisu K, Kudo K, Sasaki M, Sakushima K, Yabe I, Sasaki H, et al. 3D neuromelanin-sensitive magnetic resonance imaging with semi-automated volume measurement of the substantia nigra pars compacta for diagnosis of Parkinson's disease. Neuroradiology. 2013; 55:719–724.21. Sasaki M, Shibata E, Kudo K, Tohyama K. Neuromelanin-sensitive MRI: basics, technique, and clinical applications. Clin Neuroradiol. 2008; 18:147–153.22. Tosk JM, Holshouser BA, Aloia RC, Hinshaw DB Jr, Hasso AN, MacMurray JP, et al. Effects of the interaction between ferric iron and L-dopa melanin on T1 and T2 relaxation times determined by magnetic resonance imaging. Magn Reson Med. 1992; 26:40–45.23. Melki PS, Mulkern RV. Magnetization transfer effects in multislice RARE sequences. Magn Reson Med. 1992; 24:189–195.24. Lehéricy S, Bardinet E, Poupon C, Vidailhet M, François C. 7 Tesla magnetic resonance imaging: a closer look at substantia nigra anatomy in Parkinson's disease. Mov Disord. 2014; 29:1574–1581.25. Zecca L, Casella L, Albertini A, Bellei C, Zucca FA, Engelen M, et al. Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson's disease. J Neurochem. 2008; 106:1866–1875.26. Kashihara K, Hanaoka A, Imamura T. Frequency and characteristics of taste impairment in patients with Parkinson's disease: results of a clinical interview. Intern Med. 2011; 50:2311–2315.27. Cosottini M, Frosini D, Pesaresi I, Costagli M, Biagi L, Ceravolo R, et al. MR imaging of the substantia nigra at 7 T enables diagnosis of Parkinson disease. Radiology. 2014; 271:831–838.28. Kwon DH, Kim JM, Oh SH, Jeong HJ, Park SY, Oh ES, et al. Seven-Tesla magnetic resonance images of the substantia nigra in Parkinson disease. Ann Neurol. 2012; 71:267–277.29. Lotfipour AK, Wharton S, Schwarz ST, Gontu V, Schäfer A, Peters AM, et al. High resolution magnetic susceptibility mapping of the substantia nigra in Parkinson's disease. J Magn Reson Imaging. 2012; 35:48–55.30. Schwarz ST, Afzal M, Morgan PS, Bajaj N, Gowland PA, Auer DP. The 'swallow tail' appearance of the healthy nigrosome - a new accurate test of Parkinson's disease: a case-control and retrospective cross-sectional MRI study at 3T. PLoS One. 2014; 9:e93814.31. Blazejewska AI, Schwarz ST, Pitiot A, Stephenson MC, Lowe J, Bajaj N, et al. Visualization of nigrosome 1 and its loss in PD: pathoanatomical correlation and in vivo 7 T MRI. Neurology. 2013; 81:534–540.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Convolutional Neural Network-Based Automatic Segmentation of Substantia Nigra on Nigrosome and Neuromelanin Sensitive MR Images
- Autopy Results of Clinically Diagnosed Alzheimer's Disease
- MRI Findings in Parkinson’s Disease: Radiologic Assessment of Nigrostriatal Degeneration
- Imaging of Dopaminergic System in Movement Disorders
- Prospect of cell therapy for Parkinson's disease