J Menopausal Med.
2018 Dec;24(3):169-175. 10.6118/jmm.2018.24.3.169.
Flavonoids Fraction of Mespilus Germanica Alleviates Insulin Resistance in Metabolic Syndrome Model of Ovariectomized Rats via Reduction in Tumor Necrosis Factor-α
- Affiliations
-
- 1Cellular and Molecular Research Center, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran. p_babaei@gums.ac.ir
- 2Department of Physiology, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
- 3Department of Biology, Faculty of Basic Sciences, Urmia University, Urmia, Iran.
- KMID: 2455890
- DOI: http://doi.org/10.6118/jmm.2018.24.3.169
Abstract
OBJECTIVES
The rate of metabolic syndrome (MetS) in women diagnosed as they age is one of the main concerns of health cares. Recently new strategies used to prevent progressions of MetS toward the diagnosis of diabetes have focused on plant flavonoids. This study was aimed to investigate the beneficial effects of flavonoids fraction of Mespilus germanica leaves (MGL) on MetS in ovariectomized (OVX) rats.
METHODS
Twenty-four adult female Wistar rats, weighing 200 to 250 g, were divided into 3 groups: Sham surgery, OVX + Salin, or OVX + Flavonoid. Three weeks after ovariectomy, animals displayed MetS criteria received flavonoid injection (10 mg/kg, intraperitoneally) for 21 days. Then the body weight, body mass index, waist circumference, visceral fat, fasting blood glucose, serum insulin, lipid profiles and tumor necrosis factor-α (TNF-α) were measured.
RESULTS
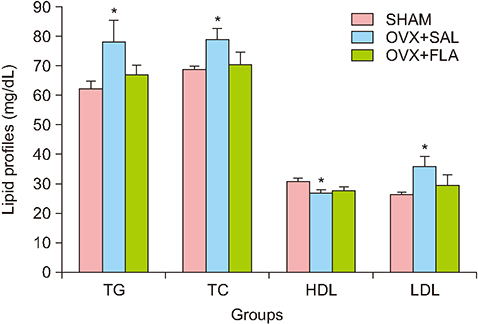
Treatment with flavonoids fraction of MGL significantly decreased serum level of insulin (P = 0.011), glucose (P = 0.024), TNF-α (P = 0.010), also MetS Z score (P = 0.020) and homeostasis model assessment of insulin resistance (P = 0.007). Lipid profiles and visceral fat showed insignificant reduction.
CONCLUSIONS
Flavonoids of MGL attenuates some of the MetS components possibly via reduction in TNF-α inflammatory cytokine.
Keyword
MeSH Terms
Figure
Reference
-
1. Lau DCW. New insights in the prevention and early management of type 2 diabetes. Can J Diabetes. 2011; 35:239–241.
Article2. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014; 2014:943162.
Article3. Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med. 2008; 168:1568–1575.4. Arthur FK, Adu-Frimpong M, Osei-Yeboah J, Mensah FO, Owusu L. The prevalence of metabolic syndrome and its predominant components among pre-and postmenopausal Ghanaian women. BMC Res Notes. 2013; 6:446.5. Tawfik SH, Mahmoud BF, Saad MI, Shehata M, Kamel MA, Helmy MH. Similar and additive effects of ovariectomy and diabetes on insulin resistance and lipid metabolism. Biochem Res Int. 2015; 2015:567945.
Article6. Krogh-Madsen R, Plomgaard P, Møller K, Mittendorfer B, Pedersen BK. Influence of TNF-alpha and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am J Physiol Endocrinol Metab. 2006; 291:E108–E114.7. Jiao K, Liu H, Chen J, Tian D, Hou J, Kaye AD. Roles of plasma interleukin-6 and tumor necrosis factor-alpha and FFA and TG in the development of insulin resistance induced by high-fat diet. Cytokine. 2008; 42:161–169.8. Luna B, Feinglos MN. Oral agents in the management of type 2 diabetes mellitus. Am Fam Physician. 2001; 63:1747–1756.9. Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011; 82:513–523.
Article10. Kasote DM, Katyare SS, Hegde MV, Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci. 2015; 11:982–991.
Article11. Banjarnahor SDS, Artanti N. Antioxidant properties of flavonoids. Med J Indones. 2014; 23:239–244.
Article12. Hoek-van den Hil EF, Keijer J, Bunschoten A, Vervoort JJ, Stankova B, Bekkenkamp M, et al. Quercetin induces hepatic lipid omega-oxidation and lowers serum lipid levels in mice. PLoS One. 2013; 8:e51588.
Article13. Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H, et al. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010; 11:1365–1402.
Article14. Annadurai T, Muralidharan AR, Joseph T, Hsu MJ, Thomas PA, Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. J Physiol Biochem. 2012; 68:307–318.
Article15. Lopes Galeno DM, Carvalho RP, Boleti AP, Lima AS, Oliveira de Almeida PD, Pacheco CC, et al. Extract from Eugenia punicifolia is an antioxidant and inhibits enzymes related to metabolic syndrome. Appl Biochem Biotechnol. 2014; 172:311–324.
Article16. Nabavi SF, Nabavi SM, Ebrahimzadeh MA, Asgarirad H. The antioxidant activity of wild medlar (Mespilus germanica L.) fruit, stem bark and leaf. Afr J Biotechnol. 2011; 10:283–289.17. Glew RH, Ayaz FA, Sanz C, Vanderjagt DJ. Changes in sugars, organic acids and amino acids in medlar (Mespilus germanica L.) during fruit development and maturation. Food Chem. 2003; 83:363–369.
Article18. Ercisli S, Sengul M, Yildiz H, Sener D, Duralija B, Voca S, et al. Phytochemical and antioxidant characteristics of medlar fruits (Mespilus germanica L.). J Appl Bot Food Qual. 2012; 85:86–90.19. Sun Y, Yu Q, Shen Q, Bai W, Kang J. Black cohosh ameliorates metabolic disorders in female ovariectomized rats. Rejuvenation Res. 2016; 19:204–214.
Article20. Prasannarong M, Saengsirisuwan V, Piyachaturawat P, Suksamrarn A. Improvements of insulin resistance in ovariectomized rats by a novel phytoestrogen from Curcuma comosa Roxb. BMC Complement Altern Med. 2012; 12:28.
Article21. Babaei P, Shirkouhi SG, Hosseini R, Soltani Tehrani B. Vitamin D is associated with metabotropic but not neurotrophic effects of exercise in ovariectomized rats. Diabetol Metab Syndr. 2017; 9:91.
Article22. Ramezani M, Darbandi N, Khodagholi F, Hashemi A. Myricetin protects hippocampal CA3 pyramidal neurons and improves learning and memory impairments in rats with Alzheimer's disease. Neural Regen Res. 2016; 11:1976–1980.
Article23. Hoseini R, Damirchi A, Babaei P. Vitamin D increases PPARγ expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition. 2017; 36:54–59.
Article24. Nounou HA, Deif MM, Shalaby MA. Effect of flaxseed supplementation and exercise training on lipid profile, oxidative stress and inflammation in rats with myocardial ischemia. Lipids Health Dis. 2012; 11:129.
Article25. Bateman LA, Slentz CA, Willis LH, Shields AT, Piner LW, Bales CW, et al. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise - STRRIDE-AT/RT). Am J Cardiol. 2011; 108:838–844.
Article26. Wegorzewska IN, Walters K, Weiser MJ, Cruthirds DF, Ewell E, Larco DO, et al. Postovariectomy weight gain in female rats is reversed by estrogen receptor alpha agonist, propylpyrazoletriol. Am J Obstet Gynecol. 2008; 199:67.e1–67.e5.27. Yang L, Wang Z, Jiang L, Sun W, Fan Q, Liu T. Total flavonoids extracted from oxytropis falcata bunge improve insulin resistance through regulation on the IKKbeta/NF-kappaB inflammatory pathway. Evid Based Complement Alternat Med. 2017; 2017:2405124.28. Li J, Gong F, Li F. Hypoglycemic and hypolipidemic effects of flavonoids from tatary buckwheat in type 2 diabetic rats. Biomedi Res. 2016; 27:132–137.29. Mahmoud MF, Hassan NA, El Bassossy HM, Fahmy A. Quercetin protects against diabetes-induced exaggerated vasoconstriction in rats: effect on low grade inflammation. PLoS One. 2013; 8:e63784.
Article30. Palacz-Wrobel M, Borkowska P, Paul-Samojedny M, Kowalczyk M, Fila-Danilow A, Suchanek-Raif R, et al. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-alpha) and interleukin-10 (IL-10) in RAW-264.7 macrophages. Biomed Pharmacother. 2017; 93:1205–1212.31. Al-Numair KS, Chandramohan G, Veeramani C, Alsaif MA. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015; 20:198–209.
Article32. Sabio G, Davis RJ. TNF and MAP kinase signalling pathways. Semin Immunol. 2014; 26:237–245.
Article33. Ye J. Regulation of PPARgamma function by TNF-alpha. Biochem Biophys Res Commun. 2008; 374:405–408.34. Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007; 48:751–762.35. Joo JK, Lee KS. Pharmacotherapy for obesity. J Menopausal Med. 2014; 20:90–96.
Article