Korean J Physiol Pharmacol.
2019 Sep;23(5):381-392. 10.4196/kjpp.2019.23.5.381.
Onion peel extract and its constituent, quercetin inhibits human Slo3 in a pH and calcium dependent manner
- Affiliations
-
- 1Department of Physiology, College of Veterinary Medicine, Chungnam National University, Daejeon 34134, Korea. kplee@cnu.ac.kr
- 2College of Pharmacy, Hanyang University, Ansan 15588, Korea.
- 3Department of Urology, Samsung Medical Center, Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Seoul 06351, Korea. drswlee@skku.edu
- KMID: 2455814
- DOI: http://doi.org/10.4196/kjpp.2019.23.5.381
Abstract
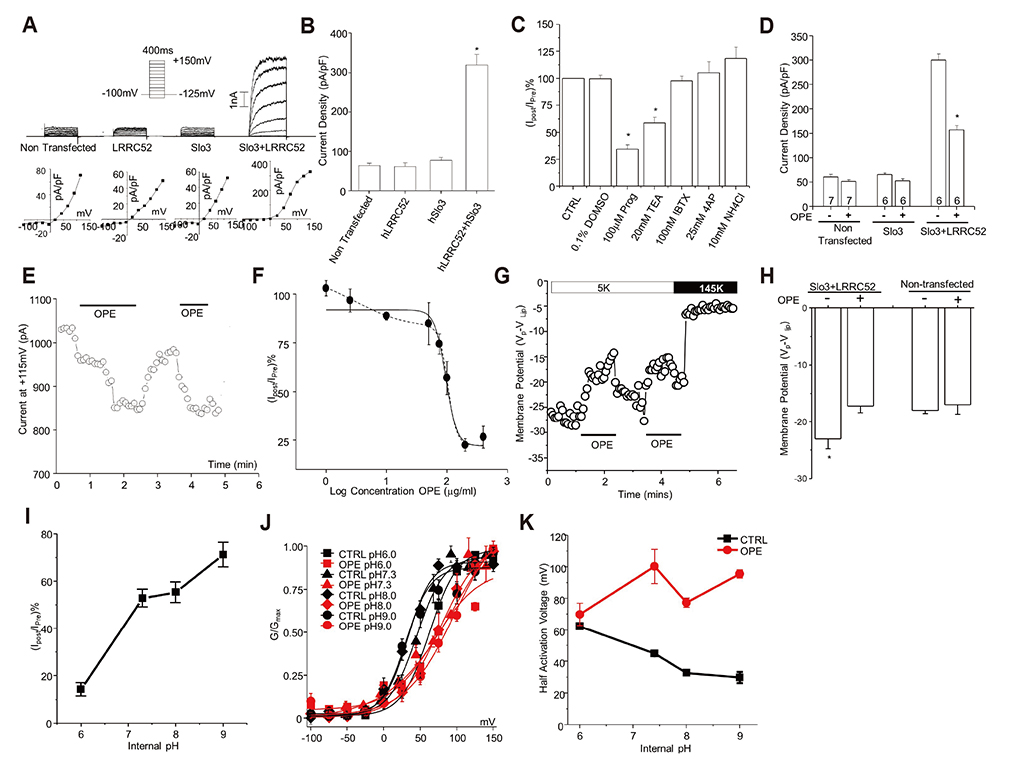
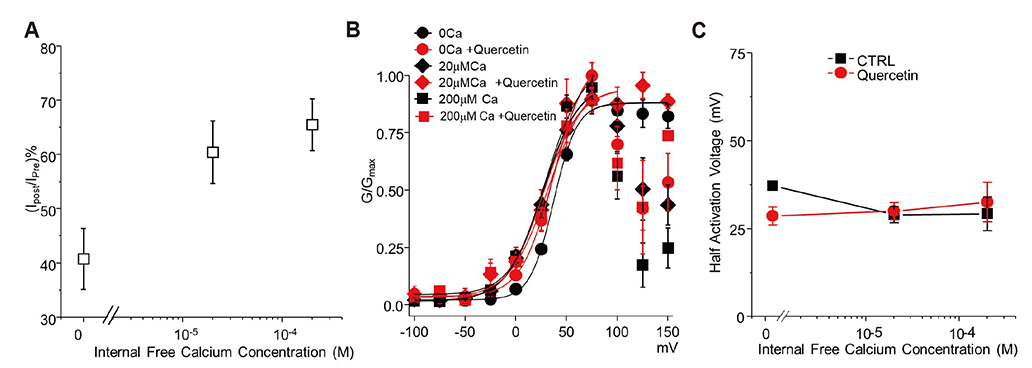
- Sperm function and male fertility are closely related to pH dependent K⺠current (KSper) in human sperm, which is most likely composed of Slo3 and its auxiliary subunit leucine-rich repeat-containing protein 52 (LRRC52). Onion peel extract (OPE) and its major active ingredient quercetin are widely used as fertility enhancers; however, the effect of OPE and quercetin on Slo3 has not been elucidated. The purpose of this study is to investigate the effect of quercetin on human Slo3 channels. Human Slo3 and LRRC52 were co-transfected into HEK293 cells and pharmacological properties were studied with the whole cell patch clamp technique. We successfully expressed and measured pH sensitive and calcium insensitive Slo3 currents in HEK293 cells. We found that OPE and its key ingredient quercetin inhibit Slo3 currents. Inhibition by quercetin is dose dependent and this degree of inhibition decreases with elevating internal alkalization and internal free calcium concentrations. Functional moieties in the quercetin polyphenolic ring govern the degree of inhibition of Slo3 by quercetin, and the composition of such functional moieties are sensitive to the pH of the medium. These results suggest that quercetin inhibits Slo3 in a pH and calcium dependent manner. Therefore, we surmise that quercetin induced depolarization in spermatozoa may enhance the voltage gated proton channel (Hv1), and activate non-selective cation channels of sperm (CatSper) dependent calcium influx to trigger sperm capacitation and acrosome reaction.
Keyword
MeSH Terms
Figure
Reference
-
1. Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010; 140:327–337.
Article2. Flesch FM, Gadella BM. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim Biophys Acta. 2000; 1469:197–235.
Article3. Baldi E, Casano R, Falsetti C, Krausz C, Maggi M, Forti G. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J Androl. 1991; 12:323–330.4. Kirichok Y, Lishko PV. Rediscovering sperm ion channels with the patch-clamp technique. Mol Hum Reprod. 2011; 17:478–499.
Article5. Lishko PV, Kirichok Y, Ren D, Navarro B, Chung JJ, Clapham DE. The control of male fertility by spermatozoan ion channels. Annu Rev Physiol. 2012; 74:453–475.
Article6. Publicover S, Harper CV, Barratt C. [Ca2+]i signalling in sperm--making the most of what you've got. Nat Cell Biol. 2007; 9:235–242.7. Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci U S A. 2011; 108:4892–4896.
Article8. Lishko PV, Miller MR, Mansell SA. The role of sperm Ion channels in reproduction. Ion Channels in Health and Disease. Elsevier;2016. p. 223–238.9. Miller MR, Mansell SA, Meyers SA, Lishko PV. Flagellar ion channels of sperm: similarities and differences between species. Cell Calcium. 2015; 58:105–113.
Article10. Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci U S A. 2007; 104:7688–7692.11. Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc Natl Acad Sci U S A. 2011; 108:5879–5884.
Article12. Mannowetz N, Naidoo NM, Choo SA, Smith JF, Lishko PV. Slo1 is the principal potassium channel of human spermatozoa. Elife. 2013; 2:e01009.
Article13. Santi CM, Martínez-López P, de la Vega-Beltrán JL, Butler A, Alisio A, Darszon A, Salkoff L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010; 584:1041–1046.
Article14. Zeng XH, Yang C, Xia XM, Liu M, Lingle CJ. SLO3 auxiliary subunit LRRC52 controls gating of sperm KSPER currents and is critical for normal fertility. Proc Natl Acad Sci U S A. 2015; 112:2599–2604.
Article15. Brenker C, Zhou Y, Müller A, Echeverry FA, Trötschel C, Poetsch A, Xia XM, Bönigk W, Lingle CJ, Kaupp UB, Strünker T. The Ca2+-activated K+ current of human sperm is mediated by Slo3. Elife. 2014; 3:e01438.16. Chávez JC, Ferreira JJ, Butler A, De La Vega Beltrán JL, Treviño CL, Darszon A, Salkoff L, Santi CM. SLO3 K+ channels control calcium entry through CATSPER channels in sperm. J Biol Chem. 2014; 289:32266–32275.17. Abi Nahed R, Martinez G, Hograindleur JP, Le Blévec E, Camugli S, Le Boucher R, Ray PF, Escoffier J, Schmitt E, Arnoult C. Slo3 K+ channel blocker clofilium extends bull and mouse sperm-fertilizing competence. Reproduction. 2018; 156:463–476.18. Shafiq S, Shakir M, Ali Q. Role of onion in the fertility issues: a review. Academ Arena. 2017; 9:40–43.19. Musavi H, Tabnak M, Alaei Sheini F, Hasanzadeh Bezvan M, Amidi F, Abbasi M. Effect of garlic (Allium sativum) on male fertility: a systematic review. J Herbmed Pharmacol. 2018; 7:306–312.
Article20. Lanzotti V. The analysis of onion and garlic. J Chromatogr A. 2006; 1112:3–22.
Article21. Chae MR, Kang SJ, Lee KP, Choi BR, Kim HK, Park JK, Kim CY, Lee SW. Onion (Allium cepa L.) peel extract (OPE) regulates human sperm motility via protein kinase C-mediated activation of the human voltage-gated proton channel. Andrology. 2017; 5:979–989.
Article22. Seifi-Jamadi A, Kohram H, Shahneh AZ, Ansari M, Macías-García B. Quercetin ameliorate motility in frozen-thawed Turkmen stallions sperm. J Equine Vet Sci. 2016; 45:73–77.
Article23. Al-Roujayee A. Improvement of sexual behavior, sperm quantity and quality by Quercetin in streptozotocin-induced diabetic erectile dysfunction. Asian Pac J Reprod. 2017; 6:6–12.
Article24. Yoshimoto H, Takeo T, Nakagata N. Dimethyl sulfoxide and quercetin prolong the survival, motility, and fertility of cold-stored mouse sperm for 10 days. Biol Reprod. 2017; 97:883–891.
Article25. Moretti E, Mazzi L, Terzuoli G, Bonechi C, Iacoponi F, Martini S, Rossi C, Collodel G. Effect of quercetin, rutin, naringenin and epicatechin on lipid peroxidation induced in human sperm. Reprod Toxicol. 2012; 34:651–657.
Article26. Taepongsorat L, Tangpraprutgul P, Kitana N, Malaivijitnond S. Stimulating effects of quercetin on sperm quality and reproductive organs in adult male rats. Asian J Androl. 2008; 10:249–258.
Article27. Silva LFMC, Araujo EAB, Oliveira SN, Dalanezi FM, Junior LRPA, Carneiro JAM, Rodriguesa LT, Hayashi RM, Crespilho AM, Dell'Aqua CPF, Dell'Aqua JA Junior, Papaa FO. Quercetin promotes increase in the fertility rate of frozen semen of Stallions considered sensitive to freezing. J Equine Vet Sci. 2018; 66:82.
Article28. Ardeshirnia R, Zandi M, Sanjabi MR. The effect of quercetin on fertility of frozen-thawed ram epididymal spermatozoa. South Afr J Anim Sci. 2017; 47:237–244.
Article29. Beazley KE, Nurminskaya M. Effects of dietary quercetin on female fertility in mice: implication of transglutaminase 2. Reprod Fertil Dev. 2016; 28:974–981.
Article30. Liang X, Xia Z, Yan J, Wang Y, Xue S, Zhang X. Quercetin inhibits human sperm functions by reducing sperm [Ca2+]i and tyrosine phosphorylation. Pak J Pharm Sci. 2016; 29:6 Suppl. 2391–2396.31. Wu SN, Chen BS, Hsu CL, Hsu TI. The large-conductance Ca2+-activated K+ channels: a target for the modulators of estrogen receptors. Curr Top Biochem Res. 2008; 10:93–101.32. Li PG, Sun L, Han X, Ling S, Gan WT, Xu JW. Quercetin induces rapid eNOS phosphorylation and vasodilation by an Akt-independent and PKA-dependent mechanism. Pharmacology. 2012; 89:220–228.
Article33. Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy. 2000; 30:501–508.
Article34. Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000; 6:909–919.35. Lindahl M, Tagesson C. Selective inhibition of group II phospholipase A2 by quercetin. Inflammation. 1993; 17:573–582.
Article36. Wijerathne TD, Kim J, Yang D, Lee KP. Intracellular calcium-dependent regulation of the sperm-specific calcium-activated potassium channel, hSlo3, by the BKCa activator LDD175. Korean J Physiol Pharmacol. 2017; 21:241–249.37. Patton C. MaxChelator [Internet]. 2014. Available from: https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator/CaEGTA-NIST.htm.38. Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, Hoyer D, Insel PA, Izzo AA, Ji Y, MacEwan DJ, Sobey CG, Stanford SC, Teixeira MM, Wonnacott S, Ahluwalia A. Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol. 2018; 175:987–993.
Article39. Leonetti MD, Yuan P, Hsiung Y, Mackinnon R. Functional and structural analysis of the human SLO3 pH- and voltage-gated K+ channel. Proc Natl Acad Sci U S A. 2012; 109:19274–19279.40. Jurasekova Z, Domingo C, Garcia-Ramos JV, Sanchez-Cortes S. Effect of pH on the chemical modification of quercetin and structurally related flavonoids characterized by optical (UV-visible and Raman) spectroscopy. Phys Chem Chem Phys. 2014; 16:12802–12811.
Article41. Tsujimoto M, Horie M, Honda H, Takara K, Nishiguchi K. The structure-activity correlation on the inhibitory effects of flavonoids on cytochrome P450 3A activity. Biol Pharm Bull. 2009; 32:671–676.
Article42. Matter WF, Brown RF, Vlahos CJ. The inhibition of phosphatidylinositol 3-kinase by quercetin and analogs. Biochem Biophys Res Commun. 1992; 186:624–631.
Article43. Wrighton DC, Muench SP, Lippiat JD. Mechanism of inhibition of mouse Slo3 (KCa 5.1) potassium channels by quinine, quinidine and barium. Br J Pharmacol. 2015; 172:4355–4363.44. Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, Kirichok Y, Ramsey IS, Quill TA, Clapham DE. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci U S A. 2007; 104:1219–1223.
Article45. Mizuno K, Padma P, Konno A, Satouh Y, Ogawa K, Inaba K. A novel neuronal calcium sensor family protein, calaxin, is a potential Ca2+-dependent regulator for the outer arm dynein of metazoan cilia and flagella. Biol Cell. 2009; 101:91–103.46. Shiba K, Baba SA, Inoue T, Yoshida M. Ca2+ bursts occur around a local minimal concentration of attractant and trigger sperm chemotactic response. Proc Natl Acad Sci U S A. 2008; 105:19312–19317.47. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, Gray AJG, Bruce L, Alexander SPH, Anderton S, Bryant C, Davenport AP, Doerig C, Fabbro D, Levi-Schaffer F, Spedding M, Davies JA. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018; 46(D1):D1091–D1106.
Article48. Chebotarev AN, Snigur DV. Study of the acid-base properties of quercetin in aqueous solutions by color measurements. J Anal Chem. 2015; 70:55–59.
Article49. Makler A, David R, Blumenfeld Z, Better OS. Factors affecting sperm motility. VII. Sperm viability as affected by change of pH and osmolarity of semen and urine specimens. Fertil Steril. 1981; 36:507–511.
Article50. Ng KYB, Mingels R, Morgan H, Macklon N, Cheong Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: a systematic review. Hum Reprod Update. 2018; 24:15–34.51. Galantino-Homer HL, Florman HM, Storey BT, Dobrinski I, Kopf GS. Bovine sperm capacitation: assessment of phosphodiesterase activity and intracellular alkalinization on capacitation-associated protein tyrosine phosphorylation. Mol Reprod Dev. 2004; 67:487–500.
Article52. Zeng Y, Oberdorf JA, Florman HM. pH regulation in mouse sperm: identification of Na+-, Cl−-, and HCO3−-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev Biol. 1996; 173:510–520.53. Khaki A, Fathiazad F, Nouri M, Khaki A, Maleki NA, Khamnei HJ, Ahmadi P. Beneficial effects of quercetin on sperm parameters in streptozotocin-induced diabetic male rats. Phytother Res. 2010; 24:1285–1291.
Article54. Azadi L, Tavalaee M, Deemeh MR, Arbabian M, Nasr-Esfahani MH. Effects of tempol and Quercetin on human sperm function after cryopreservation. Cryo Letters. 2017; 38:29–36.55. Winn E, Whitaker BD. Quercetin supplementation during boar semen thawing and incubation improves sperm characteristics [abstract]. J Anim Sci. 2018; 96:Suppl 2. 261–262. Abstract no. 489.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of onion peel water extracts on swimming endurance in rat
- Antioxidative Activity of Onion Peel Extract in Obese Women: A Randomized, Double-blind, Placebo Controlled Study
- Onion peel extract reduces the percentage of body fat in overweight and obese subjects: a 12-week, randomized, double-blind, placebo-controlled study
- The Effect of Onion Peel Extract on Inflammatory Mediators in Korean Overweight and Obese Women
- Effect of onion peel extract supplementation on the lipid profile and antioxidative status of healthy young women: a randomized, placebo-controlled, double-blind, crossover trial