Cancer Res Treat.
2016 Apr;48(2):727-737. 10.4143/crt.2014.350.
Altered Biological Potential and Radioresponse of Murine Tumors in Different Microenvironments
- Affiliations
-
- 1Department of Radiation Oncology, Yonsei University College of Medicine, Seoul, Korea. jsseong@yuhs.ac
- 2Department of Biochemistry, College of Life Science and Biotechnology, Yonsei University, Seoul, Korea.
- 3Department of Radiation Treatment Research, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 4Cancer Metastasis Research Center, Yonsei Institute for Cancer Research, Seoul, Korea.
- KMID: 2454351
- DOI: http://doi.org/10.4143/crt.2014.350
Abstract
- PURPOSE
This study was conducted to evaluate the biological features of murine hepatocarcinoma according to different tumor microenvironmental models and to determine the change in molecular and immunologic responses after radiation.
MATERIALS AND METHODS
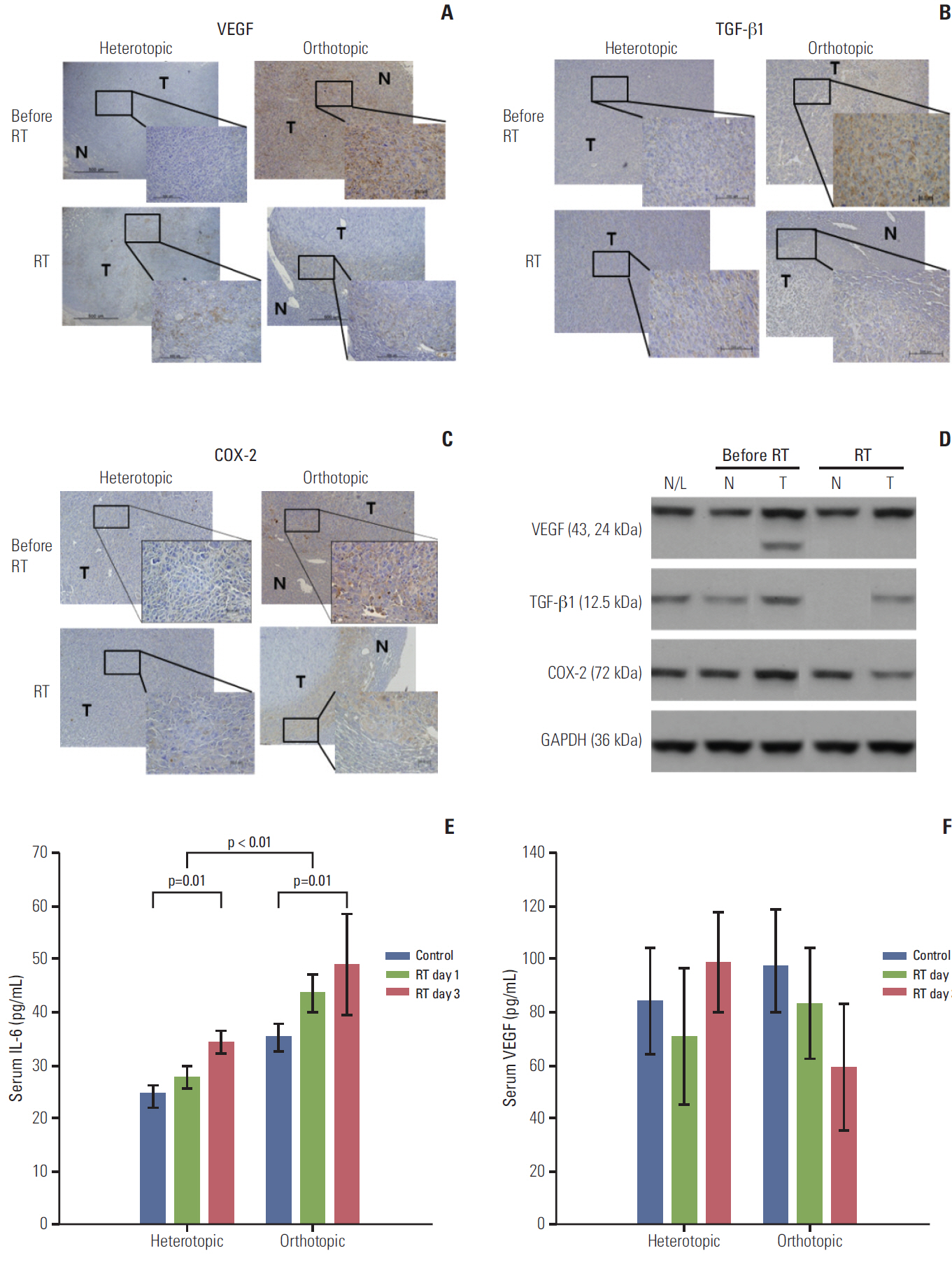
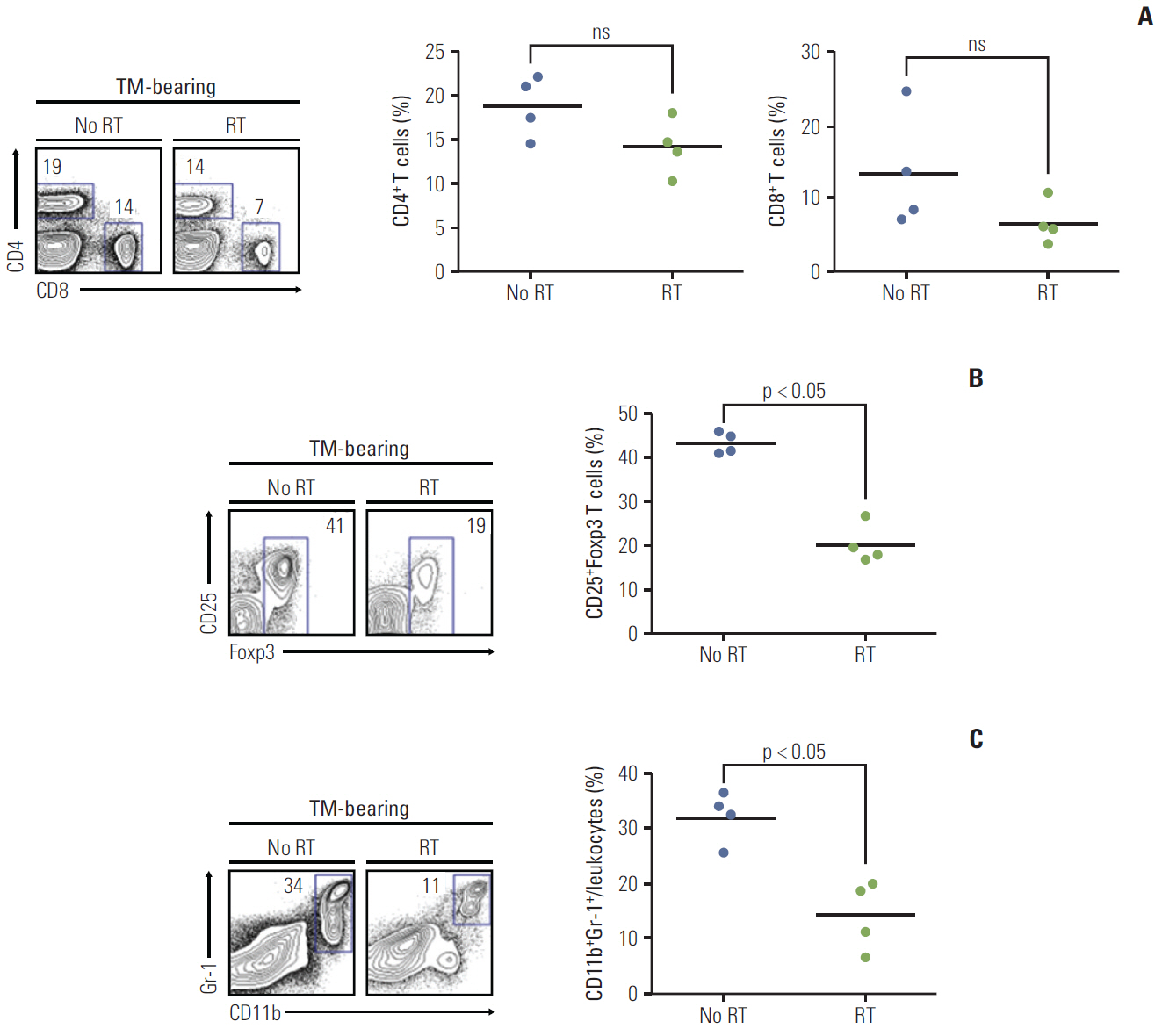
Tumor models were established in the liver (orthotopic) and thigh (heterotopic) of male C3H/HeN mice. Tumor growth and lung metastasis were assessed in these models. To evaluate the radiation effect, the tumors were irradiated with 10 Gy. Factors associated with tumor microenvironment including vascular endothelial growth factor (VEGF), cyclooxygenase-2 (COX-2), transforming growth factor beta1 (TGF-β1), CD31, and serum interleukin-6 (IL-6) were evaluated. Tumor-infiltrating regulatory immune cells, regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs) were also analyzed.
RESULTS
A higher number of lung metastases were observed in the orthotopic tumor model than in the heterotopic tumor model. VEGF, CD31, COX-2, and TGF-β1 expression was more prominent in the orthotopic tumor model than in the heterotopic tumor model. Expression of the angiogenic factor VEGF and key regulatory molecules (TGF-β1 and COX-2) decreased following radiation in the orthotopic tumor model, while the serum IL-6 level increased after radiation. In the orthotopic tumor model, the number of both Tregs and MDSCs in the tumor burden decreased after radiation.
CONCLUSION
The orthotopic tumor model showed higher metastatic potential and more aggressive molecular features than the heterotopic tumor model. These findings suggest that the orthotopic tumor mouse model may be more reflective of the tumor microenvironment and suitable for use in the translational research of radiation treatment.
Keyword
MeSH Terms
-
Angiogenesis Inducing Agents
Animals
Cyclooxygenase 2
Humans
Interleukin-6
Liver
Lung
Male
Mice
Neoplasm Metastasis
Radiation Effects
T-Lymphocytes, Regulatory
Thigh
Transforming Growth Factor beta1
Translational Medical Research
Tumor Burden
Tumor Microenvironment
Vascular Endothelial Growth Factor A
Angiogenesis Inducing Agents
Cyclooxygenase 2
Interleukin-6
Transforming Growth Factor beta1
Vascular Endothelial Growth Factor A
Figure
Reference
-
References
1. Weinberg RA. The biology of cancer. New York: Garland Science;2007.2. Oppenheimer SB. Cellular basis of cancer metastasis: a review of fundamentals and new advances. Acta Histochem. 2006; 108:327–34.
Article3. Cretu A, Brooks PC. Impact of the non-cellular tumor microenvironment on metastasis: potential therapeutic and imaging opportunities. J Cell Physiol. 2007; 213:391–402.
Article4. Tofilon PJ, Basic I, Milas L. Prediction of in vivo tumor response to chemotherapeutic agents by the in vitro sister chromatid exchange assay. Cancer Res. 1985; 45:2025–30.5. Meyn RE, Stephens LC, Ang KK, Hunter NR, Brock WA, Milas L, et al. Heterogeneity in the development of apoptosis in irradiated murine tumours of different histologies. Int J Radiat Biol. 1993; 64:583–91.
Article6. Lee EJ, Park HJ, Lee IJ, Kim WW, Ha SJ, Suh YG, et al. Inhibition of IL-17A suppresses enhanced-tumor growth in low dose pre-irradiated tumor beds. PLoS One. 2014; 9:e106423.
Article7. Kim W, Seong J, An JH, Oh HJ. Enhancement of tumor radioresponse by wortmannin in C3H/HeJ hepatocarcinoma. J Radiat Res. 2007; 48:187–95.
Article8. Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995; 36:169–80.
Article9. Park HJ, Kusnadi A, Lee EJ, Kim WW, Cho BC, Lee IJ, et al. Tumor-infiltrating regulatory T cells delineated by upregulation of PD-1 and inhibitory receptors. Cell Immunol. 2012; 278:76–83.
Article10. Gao YS, Chen XP, Li KY, Wu ZD. Nude mice model of human hepatocellular carcinoma via orthotopic implantation of histologically intact tissue. World J Gastroenterol. 2004; 10:3107–11.
Article11. Morikawa K, Walker SM, Jessup JM, Fidler IJ. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1988; 48:1943–8.12. Onogawa S, Kitadai Y, Tanaka S, Kuwai T, Kuroda T, Chayama K. Regulation of vascular endothelial growth factor (VEGF)-C and VEGF-D expression by the organ microenvironment in human colon carcinoma. Eur J Cancer. 2004; 40:1604–9.
Article13. Benoy IH, Salgado R, Elst H, Van Dam P, Weyler J, Van Marck E, et al. Relative microvessel area of the primary tumour, and not lymph node status, predicts the presence of bone marrow micrometastases detected by reverse transcriptase polymerase chain reaction in patients with clinically non-metastatic breast cancer. Breast Cancer Res. 2005; 7:R210–9.
Article14. Guang-Wu H, Sunagawa M, Jie-En L, Shimada S, Gang Z, Tokeshi Y, et al. The relationship between microvessel density, the expression of vascular endothelial growth factor (VEGF), and the extension of nasopharyngeal carcinoma. Laryngoscope. 2000; 110:2066–9.
Article15. Huang SP, Wu MS, Wang HP, Yang CS, Kuo ML, Lin JT. Correlation between serum levels of interleukin-6 and vascular endothelial growth factor in gastric carcinoma. J Gastroenterol Hepatol. 2002; 17:1165–9.
Article16. Mouthon MA, Vandamme M, van der Meeren A, Gourmelon P, Gaugler MH. Inflammatory response to abdominal irradiation stimulates hemopoiesis. Int J Radiat Biol. 2001; 77:95–103.17. Chou CH, Chen PJ, Jeng YM, Cheng AL, Huang LR, Cheng JC. Synergistic effect of radiation and interleukin-6 on hepatitis B virus reactivation in liver through STAT3 signaling pathway. Int J Radiat Oncol Biol Phys. 2009; 75:1545–52.
Article18. Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003; 22:1517–27.
Article19. Pang YL, Zhang HG, Peng JR, Pang XW, Yu S, Xing Q, et al. The immunosuppressive tumor microenvironment in hepatocellular carcinoma. Cancer Immunol Immunother. 2009; 58:877–86.
Article20. Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010; 10:170–81.
Article21. Komatsu N, Hori S. Full restoration of peripheral Foxp3+ regulatory T cell pool by radioresistant host cells in scurfy bone marrow chimeras. Proc Natl Acad Sci U S A. 2007; 104:8959–64.
Article22. Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 2013; 210:2435–66.
Article23. Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009; 1174:99–106.
Article24. Saha A, Chatterjee SK. Combination of CTL-associated antigen-4 blockade and depletion of CD25 regulatory T cells enhance tumour immunity of dendritic cell-based vaccine in a mouse model of colon cancer. Scand J Immunol. 2010; 71:70–82.25. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014; 124:687–95.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhancement of Tumor Radioresponse by Combined Chemotherapy in Murine Hepatocarcinoma
- In Vitro Radiosensitization of Flavopiridol Did Not Translated into In Vivo Radiosensitization
- Enhancement of Tumor Response by MEK Inhibitor in Murine HCa-I Tumors
- Biological Markers as Predictors of Radiosensitivity in Syngeneic Murine Tumors
- Effect of Small Dose of Radiation on Induction of Apoptosis in Murine Tumors